Advantages of Peptides
Peptides are a class of compounds formed by linking multiple amino acids through amide bonds, and their molecular size is between small molecule chemical drugs and macromolecular biological drugs. Compared with small molecule chemical drugs, peptides have higher efficacy, selectivity and specificity, and lower metabolic toxicity. Compared with macromolecular biological drugs, peptides have lower immunogenicity and can penetrate deeper into the target, and continuous production costs are lower. Based on these unique advantages, in recent years, peptide drugs have attracted more and more attention. According to statistics, the growth rate of the peptide drug market is twice that of other drugs. At present, a variety of peptide drugs have entered the market for the treatment of diabetes, osteoporosis, cancer, multiple sclerosis, chronic pain, HIV infection and many other diseases.
As we know, active peptides are the material basis for drug efficacy, so in the development process of peptide drugs, the quality control of active peptides (API) is very important, and the control of peptide-related impurities is an important work of quality control, impurities not only affect the quality and safety of clinical trial samples, in the early stage of drug discovery, These impurities may also affect the judgment of drug efficacy, and inadequate research on impurities may even lead to incorrect conclusions.
Classification of Impurities and Ways of Production
- Impurities related to the synthesis process
Chemical synthesis technology is the gold standard for peptide production. The process was initially carried out in solution, and later solid-phase peptide synthesis was introduced. After decades of continuous development, the technology has become very mature. The steps of chemically synthesizing the polypeptide include: hanging resin-deprotection group-activation and cross-linking-peptide cleavage. In this process, the steps of deprotection, activation and cross-linking are prone to impurities, which are usually points that require quality control.
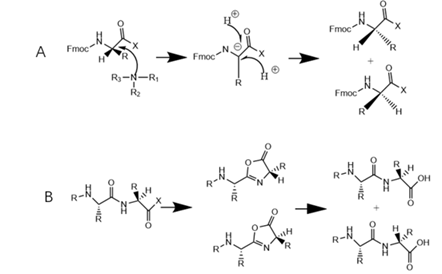
Missing peptide: As the name suggests, a missing peptide is a peptide that is missing one or more of the required amino acids, and the transient protective group of amino acids that is not completely removed, insufficient activation of the amino acid to be bound, or low cross-linking efficiency can lead to the production of the missing peptide. The identification method of the missing peptide, that is, the mass difference between the impurity and the target peptide observed in the mass spectrometry is the molecular weight of the missing amino acid minus water.
Insertional peptide: During the cross-linking process of synthetic peptides, amino acids are usually fed in excess. If the excess amino acids are not fully washed or the reaction time is too long, the amino acids will be cross-linked again to generate insertion peptides. For the identification method of the inserted peptide, the mass difference between the impurity observed in the mass spectrum and the target peptide is the molecular weight of the inserted amino acid minus water.
Misfolded peptide: It can arise when both amino acid deletions and amino acid insertions occur during a solid-phase reaction process.
Covalently attached peptide impurities: After solid phase synthesis, incomplete removal of permanent protective groups will lead to covalent attachment of protective groups to the peptide sequence, resulting in covalent attached peptide impurities. For the identification method of such impurities, if the molecular weight of the impurity in the mass spectrum differs from the molecular weight of the target peptide by 56 Da, then the attached protective group is the tBu protective group, if the molecular weight of the impurity in the mass spectrum differs from the molecular weight of the target peptide by 100 Da, then the attached protective group is the tBoc-TCS protective group.
- Degradation related impurities
Degradation related impurities include impurities formed by degradation mechanisms during manufacturing and impurities formed by degradation mechanisms during storage. The factors that cause the instability of peptide include oxidation, light, high temperature, pH, ionic strength change and adsorption, etc. The amino acid type and the position of amino acid in the peptide chain have important effects on the stability of peptide.
Epipeptide: it refers to the impurity formed by amino acid residues with one or more unexpected chiral configurations in the sequence, which may be derived from optical isomers in the starting materials, or amino acids during the synthesis by enolization or azalactonization of the chiral center. The chromatographic behavior of epi-peptide impurities is very close to that of the main component, and the molecular weight is also the same as that of the main component, so separation and identification are more difficult. Deuterated reagents can be used to hydrolyze and derivatize peptides, and the amino acids that are prone to racemization in the peptide chain can be judged by the content of chiral isomers of amino acids obtained from hydrolysis, and possible epimer peptides can be prepared by directional preparation. targeted research.
Broken peptide: Peptide bonds may be broken when peptide encounter high temperature or pH changes, resulting in broken peptides. Studies have shown that alkaline conditions are most likely to cause peptide bond breaks. The identification of the broken peptide is relatively simple, and which amide bond is broken can be inferred from the molecular weight difference between the impurity and the parent peptide.
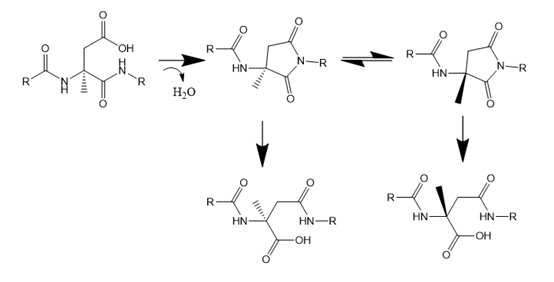
Deamidation impurities: When the peptide contains glutamine or aspartate residues, the amide bond may be removed to form glutamic acid or aspartate residues, which can be formed by two mechanisms, one is direct hydrolysis (easy to occur at pH<5), and the other is through β transformation (easy to occur under alkaline conditions), which attacks the carbonyl group on the side chain through the N atom of the amide bond in the main chain. Form a succinimide ring (a type of azalactone), which hydrolyzes under alkaline or neutral conditions to form a carboxyl group. The succinimide hydrolysis process is often accompanied by two side reactions, namely isomerization and racemization, and finally form four isomers with the same molecular weight. The identification of deamidation impurities is mainly through mass spectrometry, and the impurities detected with molecular weight +1 Da are usually deamidation impurities.
Isomerized impurity: Aspartic acid is an amino acid that is prone to isomerization. Aspartic acid is easily cyclized to the main chain due to dehydration or ammonia in the side chain to form succinimide. Succinimide has poor stability and is easy to hydrolyze. In the process of ring opening, isomers can be formed, and epi-peptide by-products can also be produced at the same time, and its formation mechanism is similar to that of asparagine deamidation.
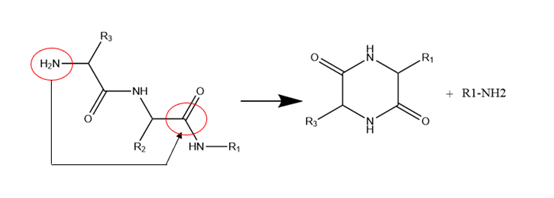
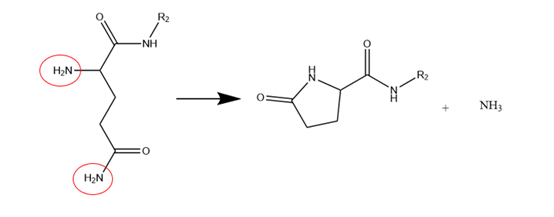
Diazinoperone and pyroglutamate: Diazinoperone refers to the N-terminal active group (usually an amino group) attacking the carbonyl group of the amide bond in the β-site main chain to form diketopiperazine analogens, resulting in a missing peptide with two amino acids, and the reaction is more likely to occur when the N-terminal amino acid hindrance is small. Pyroglutamic acid refers to the N-terminal containing glutamic acid which is cyclized to form pyrrolidone carboxylic acid. The impurity can be identified by mass spectrometry with a molecular weight of 17Da less than the parent peptide (NH3 removed).
Polymer: Intermolecular aggregation and self-association of peptides can lead to the formation of high molecular weight impurities known as polymers. There are two types of polymerization mechanisms: covalent and non-covalent. Non-covalent polymerization is formed by electrostatic or hydrophobic interaction, which is an intermediate state. When the solution concentration, pH or ionic strength changes, this force is easily weakened or disappears, and the polymerization state returns to the monomer state. when the peptide chain contains thiol or tyrosine, it can cause covalent polymerization through the formation of disulfide bonds or the formation of dityrosine. Covalent polymerization is a stable polymerization state that does not change with peptide concentration or pH, ionic strength, etc. Due to the further increase of molecular weight and volume of polymer impurities, it is easy to increase the immunogenicity and cause allergic reaction, so the content of polymer should be controlled reasonably. Polymers are usually detected by volumetric exclusion chromatography, which can be used in conjunction with mass spectrometry to determine the multiple of their polymers.
Oxidative impurities: Oxidative impurities are formed when peptides are exposed to oxygen and light during storage. Amino acids that are prone to oxidation include sulfo-containing amino acids, histidine, tyrosine, and tryptophan.
Peptide-related impurities are not only a problem that needs continuous attention in the GMP supervision during the drug development and production process, but also may cause misjudgment of developers in the early stage of drug discovery and development because of the antagonism of impurities or the super potency of the drug. Therefore, it is usually recommended to use peptides from different sources for retesting when carrying out biological tests to eliminate the influence of impurities on the drug efficacy.
References
- Muttenthaler M, King GF, Adams DJ, Alewood PF, Trends in peptide drug discovery. Nat Rev Drug Discov 20:309-325.
- V.Sanz-Nebot, I.Toro, Investigation of synthetic peptide hormones by liquid chromatography coupled to pneumatically assisted electrospray ionization msaa spectrometry:analysis of a synthesis crude of peptide triptorelin, Rapid Commun. Mass Spectrom,2001;15:1031-1039.
- Matthias D’Hondt, Nathalie Bracke, Related impurities in peptide medicines, Journal of Pharmaceutical and Biomedical Analysis 101 (2014) 2–30.
- Shikha Patel, Vivek K. Vyas, Priti J. Mehta, A Review on Forced Degradation Strategies to Establish the Stability of Therapeutic Peptide Formulation, International Journal of Peptide Research and Therapeutics (2023) 29:22.
- Brian Gregg,Aleksander Swietlow,Control Strategies for Synthetic Therapeutic Peptide APIs Part III: Manufacturing Process Considerations.
- Virender K. Sharma a& Nigel J.D. Graham , Oxidation of Amino Acids, Peptides and Proteins by Ozone: A Review, Ozone: Science & Engineering, 32: 81–90.