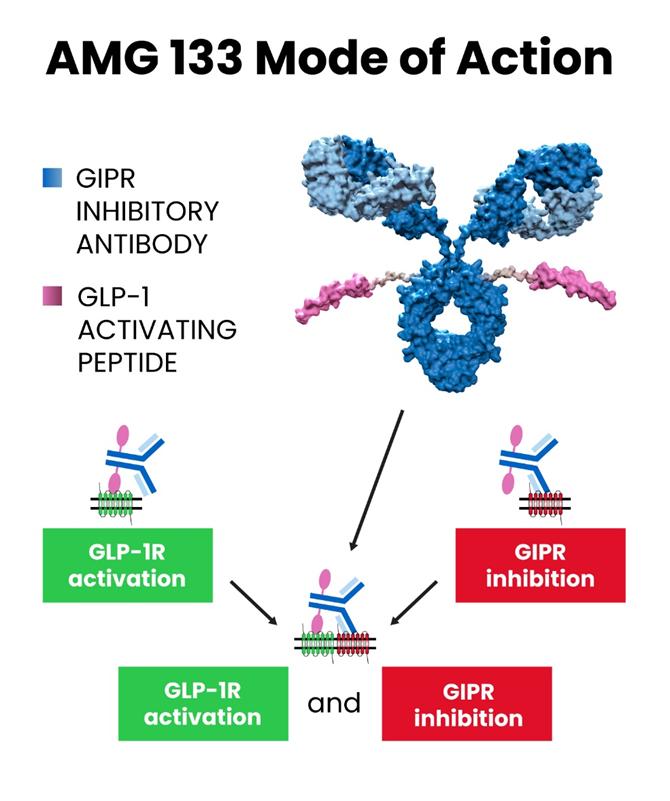
In the field of weight loss drug development, the recently disclosed clinical data of Amgen’s weight loss candidate drug Maridebart Cafraglutide (referred to as MariTide, formerly known as AMG 133) has drawn widespread attention in the industry and is considered a potential “Game Changer.” The reason why MariTide is seen as a game-changer in the weight loss drug market dominated by Wegovy and Zepbound is an important factor: MariTide’s molecular modality is a unique “antibody-peptide conjugate” (also known as Peptibody).
Antibody-peptide conjugates can be broadly classified under the well-known category of ADCs (antibody-drug conjugates). However, unlike small molecule drugs conjugated with antibodies, in the case of MariTide, peptides are conjugated. The molecular structure of MariTide involves coupling two GLP-1 receptor agonist peptides to an antibody against GIP (Glucose-dependent Insulinotropic Peptide, also known as “incretin”), forming a three-part conjugate of antibody-peptide conjugate. In this structure, the GLP-1 receptor agonist is responsible for activating the GLP-1 receptor, stimulating insulin secretion, while the anti-GIP antibody is responsible for downregulating GIP secretion. These two forces work together to achieve controlled weight loss goals.
The modality of MariTide’s antibody-peptide conjugate determines its longer half-life compared to peptides and small molecule drugs. The ongoing clinical trials involve a monthly injection frequency, and researchers are also considering extending the dosing interval to a quarter.
Structure of Peptibody
From the perspective of anti-tumor antibody-drug conjugates (ADCs), ADCs connect cytotoxic cancer drug molecules with antibodies or antibody fragments. Each component has its specific role. The antibody portion of the ADC mainly targets specific protein receptors found on tumor cells, with the goal of delivering the cytotoxic payload more directly to tumor cells, reducing damage to healthy tissues, and minimizing off-target effects. Both parts of the ADC (targeting region and payload) can be modified to target different types of tumors using different cancer drugs.
While peptide drugs have a wide range of therapeutic applications, their relatively short half-life has been a challenge, attributed to poor metabolic stability and a fluid dynamic radius below the glomerular filtration limit of the kidneys. Although the lipidation of GLP-1 peptides can enhance their binding to albumin, significantly increasing their half-life (this is currently a widely adopted pharmacokinetic enhancement strategy for GLP-1 peptides), lipidation modification is not universally applicable. Many peptide drugs require daily injections, which may affect their tolerance and patient compliance. By forming Peptibodies through conjugation with antibodies, the “tree blooming” effect can be achieved, leveraging the longer half-life of antibodies to enhance the stability of peptides.
Components of Peptibody
Peptide Segment: The peptide is one of the primary functional units of Peptibody. These peptide segments are usually designed to have specific biological activities (e.g., the GLP-1 receptor agonist in MariTide) for interaction with target molecules. The selection of peptide segments can be based on an understanding of the structure and function of the target molecule or obtained through screening large peptide libraries to identify segments with specific binding properties.
Antibody Framework: Peptibody also includes an antibody framework to provide stability and structural support. Ideally, like the anti-GIP antibody in MariTide, it not only offers structural protection but also exhibits biological activity. The antibody framework is typically composed of parts of the structure of natural antibodies but can be modified through engineering to improve Peptibody performance.
Linker: The linker is the part that connects the peptide segment and the antibody framework. The design of the linker can influence the conformation and stability of Peptibody, so careful selection is necessary to ensure Peptibody performance.
Characteristics of Peptibody
High Specificity: Peptibody can bind to specific target molecules with high specificity, a characteristic conferred by the antibody component. This specificity enhances the accuracy and effectiveness of Peptibody in both diagnostics and therapeutics.
Multifunctionality: Due to the properties of peptides and antibodies, Peptibody can be designed to have various functions, including drug delivery, targeted therapy, diagnostic labeling, and more.
Molecular Size: Peptibody is generally smaller compared to traditional antibodies. In comparison to full antibodies, Peptibody typically has a simplified structure. Although Peptibody includes parts of the antibody structure, certain large domains or functional regions are often omitted, reducing the overall size and aiding drug absorption.
Customizability: The structure and function of Peptibody can be precisely controlled through synthetic methods, allowing researchers to tailor Peptibody performance according to specific application needs.
Applications of Peptibody
Therapeutics: Peptibody can be designed for targeting and treating conditions such as cancer, autoimmune diseases, infectious diseases, and more.
Diagnostics: Peptibody can serve as diagnostic markers for detecting specific biomarkers or disease indicators.
Research: Peptibody can be employed as a research tool to study the structure, function, and interactions of biomolecules.
The First Marketed Peptibody
Romiplostim (Nplate®) is a peptibody, a fusion protein-thrombopoietin (THPO) peptide mimetic. It increases platelet count by binding and activating the human THPO receptor and is used to treat chronic idiopathic (immune) thrombocytopenic purpura (ITP). It received FDA approval on August 22, 2008.
Romiplostim is a dimeric Fc-peptide peptibody that stimulates platelet production by activating the thrombopoietin receptor. It consists of two identical single-chain subunits, each comprising 269 amino acid residues. Each subunit contains an IgG1 Fc carrier domain linked covalently to the peptide molecule. This peptide sequence includes two binding domains (epitopes) that interact with the c-Mpl receptor of the thrombopoietin receptor, each domain composed of 14 amino acids (IEGPTLRQWLAARA). It is worth noting that the amino acid sequence of romiplostim is not similar to the endogenous thrombopoietin. Romiplostim is produced through recombinant DNA technology in Escherichia coli.
As a thrombopoietin receptor agonist, Romiplostim activates cellular transcription pathways through the c-Mpl receptor (thrombopoietin receptor) to increase platelet production. Its action is similar to thrombopoietin (THPO), an endogenous glycoprotein hormone that regulates platelet production in the bone marrow.
Romiplostim, as a peptibody, exhibits inherent advantages over the peptide IEGPTLRQWLAARA, notably in the pharmacokinetic properties (plasma half-life) and significant improvement in efficacy. The improvement in half-life can be attributed to two factors:
- Increase in molecular weight (approximately 60 kD), leading to an expansion in the fluid dynamic volume beyond the threshold of glomerular filtration in the kidneys.
- Binding of Romiplostim to neonatal Fc receptors facilitates receptor-mediated recycling, preventing degradation and extending the half-life.
The average plasma half-life of Peptibody in the human body is 3 to 8 days. Although this is shorter than the half-life of certain clinical monoclonal antibodies (mAb), it represents a significant improvement compared to the administration of peptides via intravenous injection. Fc fusion is also more attractive than chemical modification because the resulting peptide can be manufactured entirely through recombinant means. Additionally, the dimerization of two Fc portions provides at least two peptides per peptibody, enhancing affinity for the target.
Latest Peptibody Pipeline
AT-04, a candidate drug co-developed by Attralus, Inc. and Ossianix, is another peptibody targeting neurodegenerative diseases such as Alzheimer’s disease. AT-04 is a peptibody that combines Attralus’ pan-amyloid removal (PAR) technology with the crystallizable fragment (Fc) portion of an IgG1 antibody. Clinical pre-data for AT-04 indicates that PAR peptide can bind to all types of amyloid proteins, including Aβ, tau, and α-synuclein, which are common pathological aggregates in neurodegenerative diseases such as Alzheimer’s.
Extracellular aggregates of Aβ amyloid protein and phosphorylated tau protein are common pathological deposits in the brains of Alzheimer’s patients. Removing Aβ amyloid plaques is a therapeutic target for Alzheimer’s disease, as seen in recent market entries like Amgen’s leqembi and the upcoming approval for donanemab. Preventing the accumulation of excessively phosphorylated tau protein may hinder the progression of Alzheimer’s disease and potentially reverse cognitive decline.
Additionally, α-synuclein is believed to play a role in Parkinson’s disease, dementia, and Lewy body diseases. Therefore, binding of AT-04 to the target proteins can induce phagocytosis, leading to the clearance of these proteins from the body. The Fc part in this process stimulates the immune system to remove amyloid proteins.
Ossianix’s VNAR antibodies (variable New Antigen Receptor antibodies, also known as shark antibodies) can further aid the brain penetration of peptibody drugs, facilitating pan-amyloid removal. The combination of VNAR antibodies with peptibody drugs has the potential to enhance their brain penetrability and therapeutic effects while potentially reducing dosage and side effects.
Future Outlook for Peptibodies
Peptibody drugs represent a significant innovation in the field of biomedicine. In the future, with increasing demand for personalized treatment and continuous technological advancements, peptibody drugs are expected to show promising prospects in various aspects.
The development of peptibody drugs will provide a more flexible and precise approach to treating various diseases. By combining the characteristics of peptides and antibodies, peptibody drugs can be designed to selectively intervene in disease targets, targeting specific biomarkers or cell surface receptors.
Furthermore, the unique structure and properties of peptibody drugs offer new possibilities in drug delivery, targeted therapy, and immune diagnostics. Through continuous innovation and technological progress, peptibody drugs are poised to bring safer and more effective treatment options for patients in the future, driving advancements in the field of biomedicine.