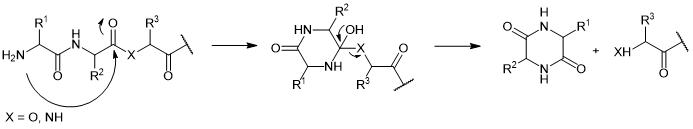
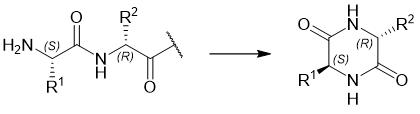
The formation of 2,5-diketopiperazine (DKP) with a bicyclic structure is one of the most detrimental side reactions and degradation pathways affecting peptide synthesis. Despite its crucial role as a versatile building block in drug discovery, the formation of DKP reactions is highly undesirable in the production of peptide drugs. DKP formation can occur during the process of peptide drug formulation, as well as in the storage of starting materials and finished products, and may even occur under solid-phase synthesis conditions.
Mechanism of DKP Formation
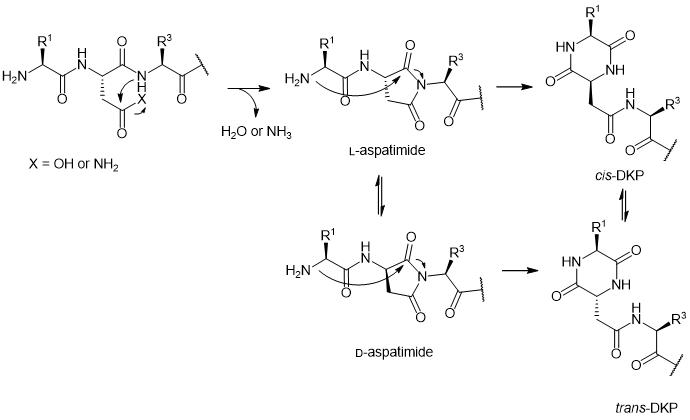
DKP can be formed through acid- or base-catalyzed reactions. The nucleophilic attack by the Nα group of the peptide on the carbonyl group between the second and third amino acid residues, either as an amide or ester carbonyl, leads to the cleavage of the amide or ester bond. The N-terminal dipeptide, as a derivative of the six-membered ring DKP, is separated from the peptide molecule. Consequently, the peptide molecule is truncated at its N-terminus by two amino acid residues, forming a byproduct characteristic of DKP. When the involved peptide is a depsipeptide (X=O in Figure 1), the formation of DKP is accelerated, as hydroxy derivatives act as better leaving groups than their amino counterparts.
FDA-approved Peptide Drugs
Considering the common occurrence of degradation in peptides with Nα groups, whether the N-terminus should be blocked to prevent DKP formation becomes a crucial consideration in peptide drug design. In fact, there are peptide drugs on the market that have a free Nα group. It is noteworthy that several peptide entities at their N-terminus are blocked through acylation, cyclization, or pyroglutamation. In peptide drugs where Nα is not blocked, they are often cyclic peptides with disulfide bonds or amide cyclization, or they may be prodrugs. Cyclization is known to effectively limit Nα activity, thereby reducing the formation of DKP. There are also three peptide drugs with free Nα, namely Golotimod, Thymodepressin, and NOV-002, which are actually derivatives modified from (L/D)-Glu side chain carboxylate salts. In these cases, the formation of DKP is also hindered.
| Peptide Name | Tradename | Amino Acid Count | N-terminal Dipeptide Structure |
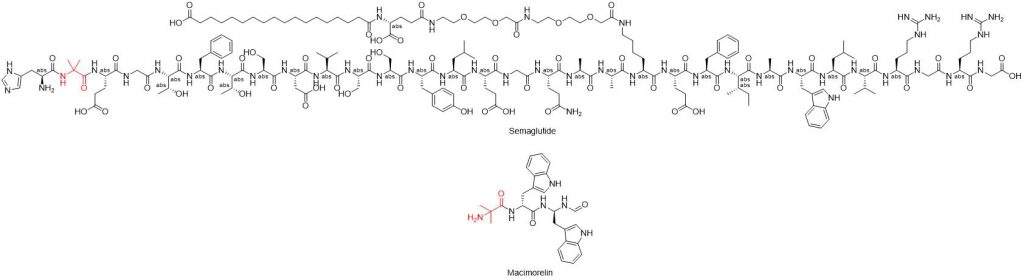
| Macimorelin | Macrilen | 3 | Aib-Trp |
| Thymopentin | Timunox | 5 | Arg-Lys |
| Semax | Semax | 7 | Met-Glu |
| Saralasin | Sarenin | 8 | Sar-Arg |
| Angiotensin II | Giapreza | 8 | Asp-Arg |
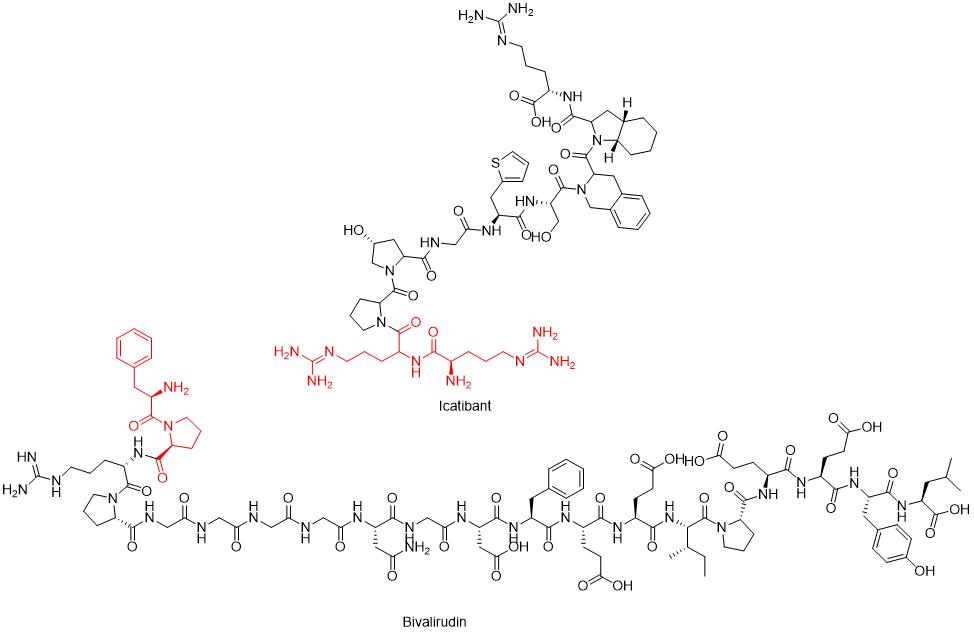
| Icatibant | Firazyr | 10 | D-Arg-Arg |
| Alloferon | Allokine-alpha | 13 | His-Gly |
| P-15 | PepGen P-15 | 15 | Gly-Thr |
| Difelikefalin | Korsuva | 5 | Phe-Phe |
Characteristics of Peptides Prone to DKP Formation
- N-terminal Secondary Amino Acid (Proline, Sarcosine)
When Pro or Sar is positioned as the second residue at the N-terminus of a peptide, the cis configuration of the peptide bond between the second and first amino acid residues is strengthened due to the properties of these secondary amino acids. This stimulation leads to DKP formation in peptides with sequences such as Phe-Pro at the N-terminus, as seen in recombinant human growth hormone (rhGH). Substituting Pro with other amino acids significantly inhibits DKP formation. N-alkyl amino acids appear at the first amino acid position in some marketed peptide drugs, such as oritavancin (N-Me-D-Val) and saralasin (Sar-Arg). It is noteworthy that despite the potential high risk of DKP with Xaa-Pro as the peptide N-terminus, approved peptide drugs like nesiritide (32-amino acid disulfide-bonded peptide) and bivalirudin (20-amino acid linear peptide) have N-terminal sequences containing H-Ser-Pro and H-D-Phe-Pro, respectively.
- Cα,α-Dialkylated Amino Acids
Cα,α-dialkylated amino acids synergistically contribute to peptide DKP formation. Although they do not promote cis amide bonds, they impose enhanced conformational restrictions on Cα-C’ and N-Cα bonds, favoring the helical secondary conformation. This conformational preference brings Nα closer spatially to the unstable amide carbonyl, promoting DKP formation. While introducing Cα,α-dialkylated amino acids may enhance susceptibility to DKP formation, some marketed peptide drugs like semaglutide (H-His-Aib, GLP-1 analog, 31 amino acids) and macimorelin (H-Aib-D-Trp) contain Aib (2-aminoisobutyric acid, Cα,α-dialkylated amino acid) residues near the N-terminus structural domain.
- N-terminal Glycine (Gly):
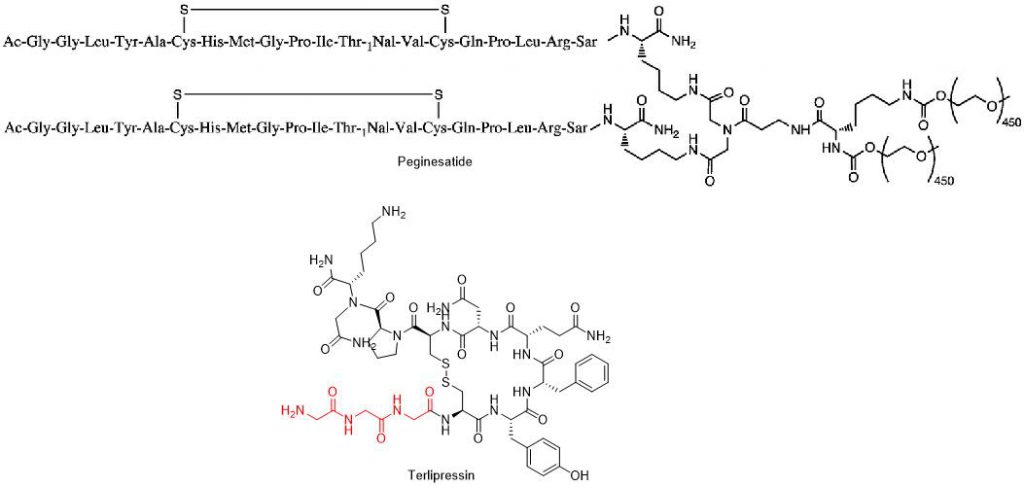
The correlation between DKP formation and N-terminal residues can be influenced by pKa, volume, amino acid configuration, and the conformational stability of DKP molecules. H-Gly-Pro-peptide is a highly sensitive N-terminal sequence that readily forms DKP due to the spatial promotion effect of Gly as the N-terminal residue and the cis configuration driven by Pro. H-Gly-Gly is another sequence highly sensitive to DKP formation. Marketed peptide drug peginesatide has a branched double N-terminal sequence Gly-Gly, but its N-terminal Gly is acetylated. Another marketed peptide drug terlipressin (cyclic peptide with disulfide bonds) has the N-terminal sequence H-Gly-Gly-Gly, and it is found to be susceptible to DKP formation during the formulation process. Peptide sequences with Gly as the N-terminal third residue also undergo DKP formation. Other marketed peptide drugs with free N-terminal Gly residues include desmopressin (H-Gly-Arg) and PepGen P-15 (H-Gly-Thr).
- N-terminal Histidine (His)
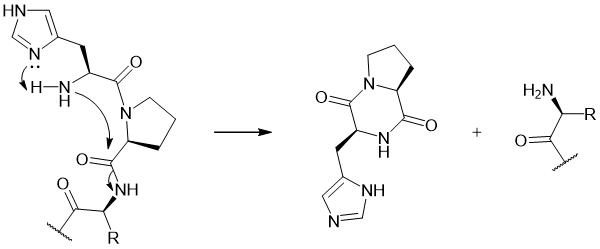
Peptides with the sequence H-His-Pro at the N-terminus are highly sensitive to DKP formation, attributed to the catalytic role of the imidazole group in the His side chain. In this structure, the His-Pro peptide bond adopts a cis conformation favorable for DKP, promoting nucleophilic attack by His-Nα on -Pro-Xaa3- main chain amide. The enhanced nucleophilicity of His-Nα is facilitated by the deprotonation of His’s basic imidazole group. This His-mediated catalytic process results in the cleavage of the Pro-Xaa3 peptide bond, ultimately forming the cyclic derivative His-Pro-DKP and complementary truncated peptide fragments.
In terms of DKP formation, the H-His-Gly sequence appears more stable than H-His-Pro. Many approved GLP-1/2 analog peptides, such as exenatide, dulaglutide, lixisenatide, and teduglutide, feature N-terminal H-His-Gly sequences. Additionally, Alloferon also has an H-His-Gly N-terminal sequence.
- Opposite Configuration of N-terminal Amino Acids
Peptide sequences composed of alternating L- and D-amino acids significantly affect DKP formation. In this case, the formed DKP molecule is stabilized thermodynamically due to the positioning of the two side chain groups, R1 and R2, on both sides of the DKP molecule plane, favoring DKP formation.
N-terminal dipeptide sequences with opposite configurations of amino acid residues are found in some marketed peptide drugs, such as icatibant (H-D-Arg-Arg) and bivalirudin (H-D-Phe-Pro).
- Disulfide-Bridged Peptides
Disulfide bonds can effectively suppress DKP formation by limiting the spatial position of the nucleophilic Nα group. This feature may explain the higher proportion of free Nα groups in marketed disulfide-bridged peptides, such as octreotide, oxytocin, and lanreotide. Compared to N-terminal unacetylated short linear peptides, they are less likely to form DKP.
- N-terminal H-Xaa-Asp Peptides
If Asp or Asn is located at the second position of the peptide N-terminus, the formation of aspartimide can promote peptide DKP formation. This aspartimide-induced DKP formation has been detected in many studies. Asn/Asp at the second N-terminal position initially forms aspartimide, and the formed aspartimide peptide intermediate can undergo differential isomerization into the corresponding non-enantiomeric isomer aspartimide or hydrolyze into Asp- or β-Asp peptides. Additionally, the aspartimide at the second N-terminal position can undergo nucleophilic attack from Nα and convert into the corresponding cis-DKP compound. Under neutral to alkaline conditions at room temperature or elevated temperatures, cis-DKP can rapidly isomerize into trans-DKP. The sensitivity of aspartimide at the second N-terminal position to DKP is attributed to the increased electrophilicity of the acyl imide part and the easy racemization of the aspartimide intermediate. In this mode, the six-membered ring of DKP is not excluded from the peptide molecules, resulting in the presence of DKP isomers in the peptides.
Plecanatide is a marketed peptide drug with both linear and Asp as the second N-terminal residue (H-Asn-Asp). Thymosin α1 has a Ser-Asp N-terminus, but its Nα group is acetylated.
Summary
- In FDA-approved short linear peptide drugs, the majority of Nα groups are acetylated and capped.
- Nα acetylation and capping can have a significant impact on the physicochemical properties of the parent peptide molecule, such as secondary structure, isoelectric point (PI), solubility, etc., particularly for short peptides.
- Long peptide drugs tend to have a relatively higher proportion of free Nα groups.
- The occurrence of unacetylated Nα is more common in disulfide-bridged peptides and amide-cyclized peptides.
- Special attention should be given to the possibility of DKP formation for short peptides containing sensitive N-terminal dipeptide sequences.