Introduction
Antigen-specific cytotoxic T lymphocytes (CTL) kill target cells by recognizing the antigen peptides bound to major histocompatibility antigen (MHC)-I molecules on the cell surface. It is one of the body’s important immune defense responses for anti-tumor, anti-graft and effective control of various infections. The MHC peptide tetramer composed of human leukocyte antigen (HLA) heavy chain and β2 microglobulin light chain, antigen peptide and dye-labeled connexin, has the characteristics of high sensitivity, specificity and efficiency. By using MHC-peptide tetramers, we can not only directly count antigen-specific T cells, but also analyze their phenotype and function, which greatly improves our understanding of the role of antigen-specific CTL in diseases.
MHC-Peptide Tetramer
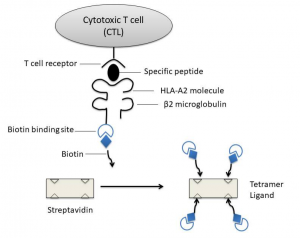
As early as 1937, Peter discovered the first histocompatibility antigen and led to the discovery of the H-2 histocompatibility antigen complex in mice. Later, a similar complex was found in the human body and named it human leukocyte antigen (HLA). These complexes in other animals are called major histocompatibility complexes (MHC). T cells recognize short peptides presented by MHC molecules through specific TCRs, so fluorescently labeled MHC-peptide complexes can be used to detect antigen-specific T cells. However, the affinity between TCR and MHC-peptide complex is very low, extremely unstable, and the dissociation time is less than 1 min[1]. The use of MHC-peptide complex multimer can increase the affinity with T cells to solve this problem. The MHC-peptide complex multimer can bind multiple TCRs at the same time, which greatly slows down the dissociation speed, thereby increasing the positive rate of detection. Altman et al. prepared MHC peptide tetramers based on this principle[2]. The light and heavy chains of MHC are produced by Escherichia coli, and they are recombined with antigen peptides by mole in vitro to form MHC-peptide complexes. After purification, a biotin molecule is connected to the C-terminus of the heavy chain of the MHC-peptide complex, then a fluorescein labeled streptavidin with four biotinylated MHC-peptide complex binds to form a tetramer (as shown Fig.1).
Figure 1: Diagram of MHC peptide tetramer: biotinylated MHC I-antigen peptide complex is mixed with fluorescently labeled avidin to form a tetramer
MHC-peptide tetramer action principle
Major histocompatibility antigens are glycoproteins located on the surface of cells. They are highly polymorphic and are antigen-presenting molecules that play a key role in immune response. The antigens that antigen-specific CTLs can recognize are mainly peptides derived from endogenous proteins and bound to MHC-I molecules. MHC-I molecules are heterodimers composed of a highly polymorphic heavy chain and a conservative light chain, β2 microglobulin [3], which are expressed in all nucleated cells. Antigen short peptides that are usually combined with MHC-I molecules are synthesized in antigen presenting cells (APC) (endogenous antigen) and binds in the peptide binding groove formed by the α1 and α2 functional regions of the heavy chain of MHC-I molecules in the endoplasmic reticulum of the cell. Subsequently, due to the non-covalent binding of β2 microglobulin to the heavy chain, MHC-I peptide complexes are more stable, and they are finally located on the cell surface. By interacting with the αβ T cell receptor (TCR) on the surface of CD8+ antigen-specific CTLs, CTLs are activated to kill infected cells.
Construction of MHC-II peptide tetramer
The basic principle is similar to the construction of MHC-I peptide tetramer. The two heavy chains of the modified MHC-II molecules are synthesized in vitro, biotinylated, and reacted with avidin to form tetramers.
Application of MHC peptide tetramer
- Quantitative analysis of specific T cells (CTLs or Th cells) that recognize a specific antigen
Tetramers are highly specific and sensitive. This feature is particularly important in the detection of certain diseases with a small number of T cells. Such as chronic hepatitis B and C virus infections, a small number of T cells not only make detection difficult, but also affect the understanding of their functions.
- Phenotypic analysis of tetramer positive cells
Combine with flow cytometry to detect multi-fluorescence markers to analyze the expression of its surface molecules, such as CD45 isomeric molecules, activation markers, homing markers CCRS, CCR7, etc., or combined with intracellular staining to detect the expression of some effector molecules or related markers, such as perforin and cell proliferation marker Ki67.
- Isolate the group of cells and perform cell function analysis (combined with other methods)
Used for the secretion and release of a specific cytokine, such as detecting the amount of secreted TNF-α to reflect the killing activity of the CTLs to be tested; or performing a cytotoxicity test to directly observe the killing function of the cell. Although this sorting of cells can also be done by magnetic beads, the use of tetramers is more economical, simple and straightforward.
- Use these cells for disease treatment and vaccine research
- Use MHC peptide tetramer/antigen-specific T cell lysis method to detect the relative activity of antigen-specific T cells
References
1.Corr, Slanetz AE, Boyd LF, Jelonek MT, Khilko S, al-Ramadi BK, Kim YS,Maher SE, Bothwell AL, Margulies DH. T cell receptor-MHC class I peptide interactions:affinity, kinetics, and specificity. Science. 1994 Aug 12;265 (5174):946-9.
2.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996 Oct 4; 274 (5284):94-6
3.Nepom Gebe J, Wang Buckner Swanson JH, Novak EJ, Reichstetter S, Reijonen H, E, Kwok WW. HLA class II tetramers: tools for direct analysis of antigen-specific CD4′ T cells. Arthritis Rheum. 2002 Jan;46(1):5-12. Review.