Vaccines are considered one of the most successful medical interventions in the past few centuries, aiming to harness the human immune system and generate lasting protection against specific diseases. Traditional vaccines rely on the use of inactivated pathogens to trigger an immune response. However, many of these formulations carry a high risk of causing allergies or autoimmune reactions. Peptide vaccines are increasingly seen as an economically efficient and safe approach to circumvent this issue.
Peptide Vaccine Development
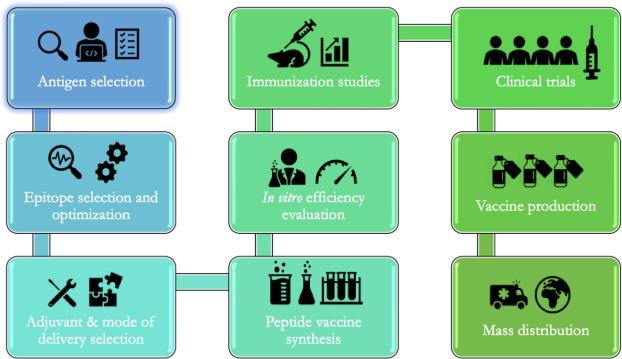
The vaccine development process is a challenging multi-step process: Antigen selection→Epitope selection→Adjuvant selection→Peptide vaccine synthesis→Immunization study→In vitro evaluation→Clinical trial.
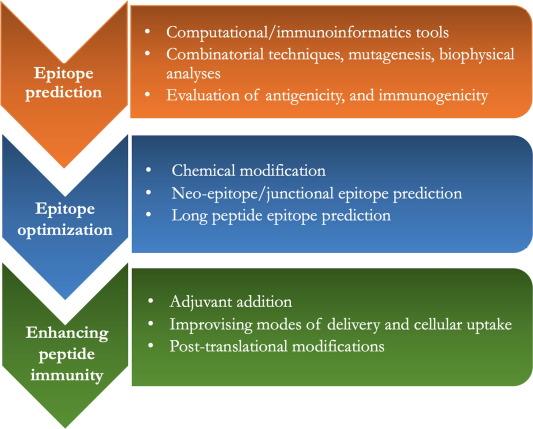
The development of peptide vaccines involves three main steps: (1) identification of immunogenic peptide epitopes; (2) optimization of peptide epitopes for improved efficacy and long-term immunity; (3) enhancing the immunogenicity of peptide epitopes using adjuvants or specific delivery systems.
- Epitope Prediction
Epitopes (or antigenic determinants) are parts of antigens recognized by immune cells. They typically consist of 5-15 amino acid residues or 1-6 sugar residues. They can be linear (continuous) or conformational, depending on their structure and interactions with complementary sites on antibodies. In linear epitopes, residues are contiguous, while in conformational epitopes, the residues that make up the epitope are folded together in the antigen’s structure. Epitope prediction is one of the initial and critical steps in the peptide vaccine design process. Various methods, including computational and immunoinformatic approaches, combinatorial techniques, mutagenesis, biophysical methods, and binding analysis, are used to identify epitopes.
Various databases and algorithms are available for selecting and identifying candidate epitopes, significantly reducing the time required for experimental epitope prediction. In these computer-based methods, the primary amino acid sequence or 3D structure of antigen proteins from the target pathogen is used as input to analyze the existence of sequences (epitopes) capable of binding to major histocompatibility complex (MHC). In humans, MHC is referred to as human leukocyte antigen (HLA).
- Assessing Epitope Antigenicity and Immunogenicity
The predicted immunogenicity of peptide epitopes can be analyzed through two in vivo methods: 1) in vivo immunogenicity analysis using HLA transgenic mice; 2) antigenicity testing using human antigen-presenting cells (APCs).
- Epitope Optimization
Activation of cytotoxic T lymphocyte (CTL) immune responses does not require a specific conformation of epitopes (they are linear). In contrast, B cell activation requires epitopes to have a conformation similar to their native antigen. Over 90% of B cell epitopes are discontinuous, and they need to maintain their native structure to bind to antibodies generated during B cell activation. To maintain this conformation, epitopes are sometimes chemically modified or extended in length. For example, peptide locking into an α-helix conformation can be achieved by introducing a linker between side chains of amino acids that are distant. Introducing artificial chemical bonds between different amino acid side chains in a peptide can fold the peptide into the desired conformation. Another method that helps promote the native conformation is adding extra auxiliary sequences at the sides of epitopes, such as linking the yeast protein GCN4 to the C and N termini of short peptides, promoting the formation of an α-helical structure in epitopes.
Pan HLA-DR-binding epitope (PADRE) is an auxiliary peptide used to stimulate antibody-mediated B cell responses and has demonstrated safety and efficacy in multiple clinical trials. PADRE consists of D-alanine, L-cyclohexylalanine, and aminohexanoic acid. Another strategy to enhance the peptide-MHC binding affinity is to stabilize peptides by adding β-amino acids to the epitopes.
Enhancing Peptide Vaccine Immunity
Improving the immune efficacy of peptide vaccines can be achieved by modifying peptide size or binding properties, directly activating APCs or co-administering with adjuvants, or using effective peptide delivery systems to enhance internalization and phagocytosis. Introducing post-translational modifications to peptide vaccines may also further enhance their effectiveness.
- The Use of Adjuvants
Adjuvants are substances co-administered with vaccines to stimulate antigen presentation and enhance immune efficacy. They reduce the antigen dose, induce rapid induction, and sustain B and T cell persistence. Most adjuvants induce local inflammation at the site of administration, recruiting various cytokines and chemokines and stimulating CCR7 expression on APCs to facilitate antigen migration to lymph nodes. The most common adjuvants are Toll-like receptor (TLR) agonists, C-type lectin receptor (CLR) agonists, and nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) agonists.
Adjuvants of plant origin, such as oligosaccharides and inulin, can induce two types of immune responses in humans. Polyactin A, a synthetic peptide with multiple arginine residues, has been used as an effective adjuvant for peptide cancer vaccines. The most common carbohydrates and derivatives used as adjuvants include mannose, glucan, chitosan, hyaluronic acid, saponins, alpha-galactosylceramide, and the polymerized form of muramyl dipeptide (MDP).
Peptide Vaccine Indications
- Influenza
Due to the significant sequence diversity and high mutation rate of influenza viruses, the development of effective influenza vaccines is challenging. One target is the influenza hemagglutinin (HA), a glycoprotein family that allows the virus to enter host cells. To mitigate sequence diversity, vaccine design focuses on highly conserved domains, especially those targeted by broadly neutralizing antibodies on viral envelope glycoproteins. Due to the high conservation of the backbone region of HA2, it represents an excellent target.
- Hepatitis Viruses
Infection with hepatitis C virus (HCV) can lead to liver diseases such as cirrhosis or hepatocellular carcinoma. The development of effective HCV vaccines has been hindered by the variability of sequences within T cell-targeted epitopes. Most antibodies against HCV react with epitopes in the E2 glycoprotein. A novel peptide vaccine, IC41, containing five synthetic peptides with HCV T cell epitopes and the adjuvant poly-L-arginine, has been developed. However, T cell responses were modest and did not significantly differ in HCV RNA in most patients, necessitating further optimization.
- HIV/AIDS
Therapies against HIV primarily rely on small molecule antiretroviral drugs, which can significantly improve disease management. For example, the 36-amino acid peptide enfuvirtide (brand name Fuzeon®) inhibits the fusion of the gp41 HIV viral envelope protein with cell membranes, preventing viral entry into cells. From a vaccine perspective, the V3 loop of the gp120 envelope protein of HIV-1 (HIV envelope variable region 3, V3) has been identified as a candidate for peptide-based vaccine design as it is a target for neutralizing antibodies.
- SARS-CoV-2 and Related Coronaviruses
The global COVID-19 pandemic caused by the SARS-CoV-2 virus has spurred the rapid development of vaccines based on mRNA and adenovirus vectors, among others. However, peptide-based SARS-CoV-2 vaccines have not yet been deployed. Sequencing of SARS-CoV-2 proteins has now been completed, and it is possible to identify key regions involved in the binding of its spike protein to target cells, representing potential targets for therapeutic interventions.
- Cancer Immunotherapy
Immunotherapy for treating cancer is a major research focus, aiming to activate the immune response against cancer cells’ immune evasion mechanisms. The primary focus is on modulating T cell responses as these cells are capable of clearing tumors. Cancer cells are characterized by the overexpression or mutations of certain proteins, making them targets for immunotherapy. Prominent tumor-associated antigens include mucin-1 (MUC1), human epidermal growth factor receptor 2 (HER-2), cancer-testis antigen 1 (NY-ESO-1), T cell-recognized melanoma antigen 1 (Melan-A or MART-1), prostate-specific antigen (PSA).
Eradicating invasive fungal infections often requires months to years of continuous drug administration, increasing the risk of drug-resistant variants. Additionally, many emerging fungal diseases are caused by multidrug-resistant fungal strains. Beyond these obstacles, the number of antifungal drugs approved for treatment is limited, and many of them are known to cause severe side effects.
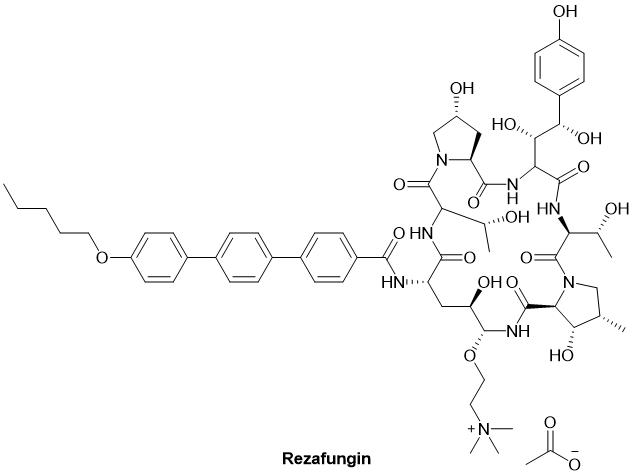
Despite these challenges, there was a breakthrough in antifungal peptide drugs in the first quarter of 2023. On March 22, 2023, the FDA approved Rezzayo™ (intravenous rezafungin) for the treatment of candidemia and invasive candidiasis in patients aged 18 and above. Rezafungin also became the first new echinocandin to receive FDA approval in over a decade. Rezafungin is a hexapeptide that inhibits the 1,3-β-D-glucan synthase complex in the fungal cell wall, capable of destroying the cell wall of various fungal species, including Candida. While not a peptide vaccine, the role of peptides in the antifungal process has received significant attention in the industry.
Advantages and Limitations of Peptide Vaccines
The use of peptides allows vaccines to target very specific epitopes while minimizing the risk of allergies or autoimmune reactions. Besides the advantages in terms of specificity and safety, peptide vaccines offer clear cost and supply advantages by reducing production costs and delivery times.
However, the development of vaccines based on these minimal sequences is not without challenges. Peptides often lack immunogenicity, meaning they may not induce a strong or long-lasting immune response. Additionally, the pharmacokinetics of these molecules determine their instability and suboptimal bioavailability, as they are prone to widespread protease degradation before successfully triggering an immune response.
Over the past few decades, scientists have devised numerous strategies to overcome these limitations. Peptide conjugation has been one of the most successful strategies to date. For example, peptides can be conjugated with proteins, such as KLH or BSA, which enhances their stability and immunogenicity. Another strategy involves the development of multimeric peptide vaccines.
In summary, the advantages of peptide vaccines include:
- Safety: Peptide vaccines are generally considered safe, as they are composed of short amino acid chains and do not contain live or attenuated pathogens. They pose minimal risk of inducing the disease they target, even in individuals with weakened immune systems, addressing potential safety concerns seen with some viral vector vaccines.
- Specificity: Peptide vaccines offer high specificity. They can be precisely designed to target specific pathogens, strains, or variants. This specificity is especially crucial for diseases caused by rapidly evolving pathogens, such as influenza and HIV.
- Reduced Risk of Adverse Effects: Due to the absence of potential reactogenic components, peptide vaccines are less likely to cause adverse reactions or side effects.
- Ease of Production: Compared to traditional vaccines, peptide vaccine production is relatively straightforward and cost-effective, especially when dealing with rapidly changing pathogens. The production process can be standardized without the need for complex live culture methods.
- No Reversion Risk: Live attenuated vaccines carry a risk of reversion to a virulent form. Peptide vaccines do not pose this risk, as they do not contain live pathogens.
- Stability: Peptide vaccines are generally stable and have longer shelf lives compared to some other vaccine types, such as live vaccines requiring strict cold chain storage.
- No Pathogen Escape Risk: Unlike live vaccines, peptide vaccines do not carry the risk of pathogen mutations or evolution to evade vaccine-induced immunity.
- Reduced Cross-Reactivity: Peptide vaccines can minimize cross-reactivity with host tissues, lowering the risk of autoimmune reactions.
- Immunological Monitoring: Since specific epitopes are known, peptide vaccines typically offer a more direct way to monitor and measure immune responses. This can aid in evaluating vaccine efficacy.
The limitations of peptide vaccines primarily manifest in the following ways:
- Limited Immunogenicity: Peptides themselves may have limited immunogenicity compared to whole proteins or live attenuated vaccines. They may not induce a strong or long-lasting immune response, potentially necessitating booster doses.
- HLA Restriction: Peptide vaccines are typically presented to the immune system through HLA. Different individuals have different HLA types, and not all peptides can efficiently bind to all HLA types. This can limit the broad applicability of peptide vaccines and may require personalized approaches in some cases.
- Epitope Identification: Identifying the most immunogenic and conserved epitopes for a given pathogen can be a complex and time-consuming process, often requiring extensive research and sequencing.
- MHC Haplotype Coverage: Achieving broad population coverage may necessitate designing vaccines that cover various MHC haplotypes, adding complexity to vaccine development.
- Incomplete Protection: Peptide vaccines may not provide as comprehensive protection as whole-pathogen vaccines. For example, they may be less effective at preventing mucosal surface infections, which is linked to their limited immunogenicity.
- Adjuvant Requirements: In some cases, peptide vaccines may require adjuvants or delivery systems to enhance their immunogenicity, increasing the complexity of vaccine formulations.
- Epitope Diversity: Some pathogens have multiple epitopes, making it challenging to select the most effective ones for peptide vaccines.
References:
- Kalita, P. et al., Methodological advances in the design of peptide-based vaccines, Drug Discovery Today, 2022, 27, 1367-1380.
- Wang, W. et al., Polyactin A is a novel and potent immunological adjuvant for peptide-based cancer vaccine, Int Immunopharmacol, 2018, 54, 95-102.
- Lalezari, J. P. et al., TORO 1 Study Group Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med., 2003, 348, 2175- 2185.