Creative Peptides has established an advanced surface plasmon resonance (SPR) platform with several applications for drug discovery, including fragment-based screening, targets characterization, lead optimization, and many other services that customer requires. This technique enables real time study of the interaction between immobilized receptors and analytes in solution without labelling of the analyte.
SPR is an extraordinary sensitive method for the detection of molecular interactions without lablelling. In general, binding response includes three phases: association, equilibrium and dissociation. Based on these data, scientist will use the mathematic model to calculate the association (Ka) and dissociation (Kd) rate constants and ultimately determine the binding affinity (KD). Different from isothermal calorimetry (ITC) that measures antibody binding affinity based on equilibrium point, SPR is able to obtain full kinetic parameters to evaluate all the effects of association and dissociation as well as antibody affinity.
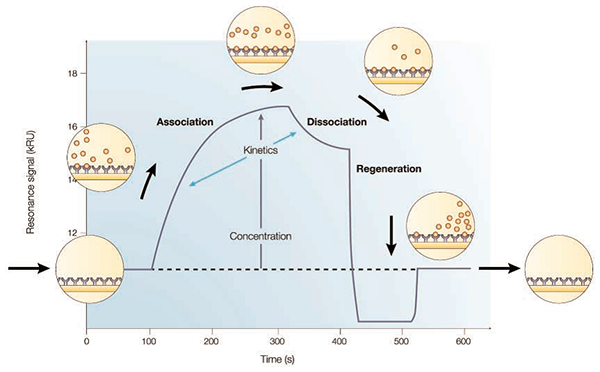
In other hand, using a SPR biosentor to investigate blocking offers several advantages over enzyme-linked immunosorbent assay (ELISA), which requires labels and provides only an end-point analysis. Indeed, epitope binding via ELISA is often hindered by the inability of finding a secondary reagent that can detect one mAb selectively over another when mAbs are competed against one another in pairs. Although biotinylating or reformatting a mAb may distinguish it from other mAbs in a test panel, it can make sample preparation tedious and unsuitable for performing high-throughput screening in a combinatorial-type format. Furthermore, ELISA may be unfeasible if the plated reagent is precious and/or expensive. In contrast, SPR biosentor reveals the entire binding profile between an interacting pair of molecules without labels, and the ligand-coated surfaces and/or solution binding partners are able to be reused.
Antigen-Antibody interaction by SPR to test the possibility of several different monoclonal antibodies binding simultaneously to an antigen. A MAb is immobilized in a matrix and then the antigen and a sequence of MAbs are injected in turn. A complete epitope map is obtained by changing the sequence of antibodies until all combinations have been tested.
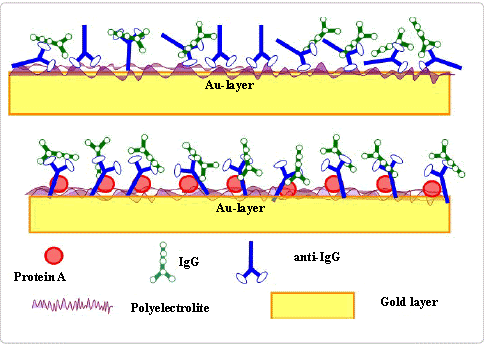
Antigen-Antibody interactions by SPR also provide a high-throughput way to test the possibility of monoclonal antibodies binding to an antigen. The analyzed antibodies are first been captured by high-affinity anti-IgG antibody immobilized on the sensor chip surface. Then a series of concentrations of antigens are sequentially injected across the surface. Based on the sensorgram curve, people can rank the antibodies according to the kinetic parameters and select monoclonal antibodies with the best characters of your preference.
Creative Peptides has extensive experience in providing One-stop-shop SPR services from synthesis of peptide antigen to SPR service. For more detailed information, please feel free to contact us at Email or directly sent us an inquiry.
SPR-based antigen-antibody interaction analysis enables real-time monitoring of binding events without labeling. It provides kinetic and affinity data describing molecular recognition behavior.
SPR allows direct observation of association and dissociation processes. This enables comprehensive kinetic characterization beyond equilibrium-only measurements.
SPR delivers full binding profiles in real time without secondary reagents. ELISA typically provides end-point signals and may be limited in competitive binding formats.
SPR analysis generates association rate, dissociation rate, and binding affinity values. These parameters support detailed comparison of interaction strength and stability.
Yes, SPR supports high-throughput comparison of multiple antibodies against a single antigen. Antibodies can be ranked based on kinetic performance and binding profiles.
Epitope mapping is achieved by sequential injection of antibodies over antigen-bound surfaces. Competitive and non-competitive binding patterns reveal epitope relationships.
Peptide antigens are well suited for SPR when immobilized with controlled orientation. They enable focused analysis of defined binding regions.
SPR assay design can be optimized for peptides, proteins, or antibody capture strategies. Customization ensures data relevance across diverse interaction studies.
