As a leading supplier of bioactive peptides, we provide comprehensive services for glycosylated peptide development and production from mg to kg quantities to meet your project needs.
Glycosylation is the most common post-translational modification of proteins in eukaryotic cells. As one of the most important recognition signals, glycosylated proteins and peptides are involved in much intracellular communication, such as apoptosis, cell growth regulation, infection process, and immunological differentiation.
Protein glycosylation is an important protein post-translational modification process that refers to the attachment of sugar molecules (called glycans or glycosides) to specific amino acid residues by covalent bonds. Glycosylation of protein occurs in the endoplasmic reticulum and Golgi apparatus and is catalyzed by glycosyltransferases and glycosidases. Glycosylation is one of the most common and complex post-translational modifications in eukaryotes, and about half of all mammalian proteins are involved in glycosylation.
Protein glycosylation is important to proteins' structures and activities. Glycosylation keeps proteins stable and prevents denaturing in extreme heat, acidic environment. Moreover, these sugar chains prevent the proteins from collapsing among themselves, and thus proteins keep folding and operating normally. Glycosylation also controls protein three-dimensionality and thus charge and bioactivity.
Glycosylation is also involved in cell signaling, cell detection, immune response and cell proliferation. For instance, glycoproteins signal cells and identify cells through their binding to cell surface receptors. Glycosylation also satisfies the immune response in the immune system (there are even some sugar chains that function as special pathogen receptors and enable pathogens to attack host cells without detection by the immune system).
However, glycosylation of proteins is also involved in disease development. Pathologies ranging from inflammatory conditions, to rheumatoid arthritis, Alzheimer's and cancer, to name a few, were associated with aberrant glycosylation patterns. For instance, in cancer cells, a wrong glycosylation might lead to cancer cell growth, migration and spread. Hence the research on protein glycosylation and its dynamics is both an aid to unravelling many cell functions and disorders, and a disease biomarker and drug target.
Protein glycosylation can be categorized into four main categories mainly according to various glycosidic linkages between the amino acid and the sugar, including
N-linked glycosylation is an important post-translational modification of proteins, mainly in eukaryotes. It involves the attachment of N-glycan chains to asparagine (Asn) residues of proteins via N-glycosidic bonds, usually on a specific sequence pattern Asn-X-Ser/Thr (where X represents any amino acid other than proline). The process of N-linked glycosylation begins at the endoplasmic reticulum (ER), in which an oligosaccharide block (Glc3Man9GlcNAc2) consisting of N-acetylglucosamine (GlcNAc), mannose (Man), and glucose (Glc) is transferred to the asparagine residue of the nascent peptide chain. This oligosaccharide block is then further modified in the ER and Golgi apparatus to form three main types of N-glycan chains: high mannose, heterozygous, and complex. N-linked glycosylation is essential for protein folding, stability, cellular localization, and multiple biological processes such as cell adhesion, proliferation, and signaling. In addition, N-linked glycosylation has also been implicated in the development of cancer, as its dysfunction may lead to the development of the disease.
O-linked glycosylation is a protein post-translational modification that mostly occurs in eukaryotic cells. In this modification, oligosaccharides form covalent bonds with the hydroxyl groups of serine (Ser) or threonine (Thr) residues through oxygen atoms. Glycosylated proteins typically involve N-acetylgalactosamine (GalNAc) as the core unit, but can also be other types of sugars such as N-acetylglucosamine, mannose, glucose, xylose (Xyl), and fucose (Fuc). O-linked glycosylation takes place in the Golgi apparatus of the cell and does not require a single precursor oligosaccharide unit to be transferred directly to the nascent peptide chain, but rather directly on folded or folded proteins. This form of glycosylation differs from N-linked glycosylation, which requires the transfer of a precursor core unit to a nascent peptide chain and is completed in the endoplasmic reticulum and Golgi apparatus. O-linked glycosylation plays an important role in many biological processes, such as protein folding, stability, enzyme sensitivity, and function. In addition, O-linked glycosylation affects protein secretion, immune responses, and cell-to-cell interactions. In some cases, O-linked glycosylation has also been implicated in the development of diseases such as inflammatory bowel disease and cancer.
C-mannosylation is a protein glycosylation change whereby a mannose molecule is attached to the C2 residue of a tryptophan with α-C-glycosidic bond. This glycosylation process typically occurs in the endoplasmic reticulum and is catalyzed by C-mannose transferase. The consensus sequence of C-mannosylation is Trp-Xxx-Xxx-Trp, i.e., modified on the first tryptophan residue. C-mannosis is observed in a variety of cell types and is found in mammals, Caenorhabditis elegans, amphibians, and birds, but not in E. coli or yeast. This form of glycosylation plays an important role in protein stability, folding, and secretion. For example, C-mannosylation can increase the structural stability of certain proteins, such as human RNase 2. In addition, C-mannosylation may also affect the enzymatic activity of proteins, for example, it can promote the enzymatic activity of ADAMTS4 and lipoprotein lipase, while inhibiting the enzymatic activity of HYAL1. It is important to note that C-mannosation is not limited to mammals and is also present in certain parasites, such as Plasmodium and Toxoplasma gondii. The DPY-19 homologous proteins in these parasites are able to C-mannan glycation of the adhesin of the TRAP/MIC2 family, thereby affecting the virulence of the parasite and the invasion of host cells. Although the specific role of C-mannosylation in cell signaling is not fully understood, it has been suggested that it may enhance the activation state of macrophages by affecting the LPS signaling pathway. In addition, C-mannosylation has also been found to modulate the Wnt/β-catenin signaling pathway, thereby affecting the migration ability of cancer cells.
Glycosylphosphatidylinositol anchoring is an important post-translational modification through which many proteins are able to bind to the outer surface of the cell membrane. GPI-anchored proteins are a class of key structural proteins in eukaryotic cells, which are anchored to the cell membrane through glycosylphosphatidylinositol and are involved in a variety of biological processes, such as signaling, cell recognition, and immune response. The biosynthesis of GPI-anchored proteins is mainly carried out in the endoplasmic reticulum. First, GPI precursors are progressively synthesized on the outer membrane of the ER, including the addition of sugars and ethanolamines. The final GPI precursor then binds to a protein with a GPI-attachment signal peptide to form a monolithic structure. This signal peptide is usually located at the C-terminus of the protein, and its hydrophobicity determines the localization of the protein in the ER.
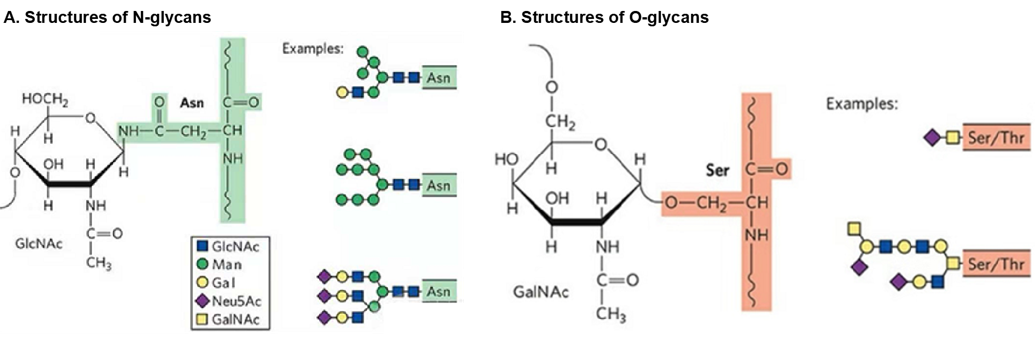 Fig.1 Structures of N-glycans and O-glycans. (Nelson, David L., et al., 2008)
Fig.1 Structures of N-glycans and O-glycans. (Nelson, David L., et al., 2008)
Creative peptides recognizes that all our clients have specific and unique requirements. We provide common monosaccharide, disaccharide and polysaccharide services. Other glycosylation modifications (sialic acid, fucose, etc.) can also be performed on the main chain and side chain of the polypeptide.
Glucose
Glucose is one of the main substrates for protein glycosylation and is of great significance for protein function and biological processes. Through the action of glycosyltransferases, glucose can form glycosidic bonds with amino acid residues on proteins, resulting in the formation of glycoproteins. This glycosylation modification can significantly affect the structure, function, and intracellular distribution and stability of proteins.
| Name | CAS | Formula | Acetate Groups Removed |
|---|---|---|---|
| Fmoc-L-Ser((Ac)3-β-D-GlcNAc)-OH | 160067-63-0 | C32H36N2O13 | Yes&No |
| Fmoc-L-Thr((Ac)3-β-D-GlcNAc)-OH | 160168-40-1 | C33H38N2O13 | Yes&No |
| FMoc-Asn(β-D-GlcNAc(Ac)3)-OH | 131287-39-3 | C33H37N3O13 | Yes&No |
| beta-D-Glucose pentaacetate | 604-69-3 | C16H22O11 | Yes&No |
| Gluconic acid | 526-95-4 | C6H12O7 | NO |
| 6-phosphogluconic acid | 921-62-0 | C6H13O10P | NO |
| 2,3,4,6-TETRA-O-ACETYL-BETA-D-GLUCOPYRANOSYL ISOTHIOCYANATE | 14152-97-7 | C15H19NO9S | Yes&No |
Galactose
The significance and importance of galactose in protein glycosylation is reflected in many aspects. In cellular metabolism, galactose promotes the repair of the glycosylation pathway. For example, in UGP-deficient cells, the addition of galactose can improve the glycosylation efficiency of these cells, suggesting that galactose plays an important role in maintaining normal glycosylation processes. In addition, galactose is also involved in the regulation of immunoglobulin function, such as the Fc core galactosylation of IgG can enhance antibody-dependent cytotoxicity (ADCC) effects.
| Name | CAS | Formula | Acetate Groups Removed |
|---|---|---|---|
| Fmoc-L-Ser((Ac)3-β-D-GalNAc)-OH | 1676104-71-4 | C32H36N2O13 | Yes&No |
| Fmoc-L-Ser((Ac)3-α-D-GalNAc)-OH | 120173-57-1 | C32H36N2O13 | Yes&No |
| Fmoc-Thr(GalNAc(Ac)3-α-D)-OH | 116783-35-8 | C33H38N2O13 | Yes&No |
| Fmoc-L-Thr(β-D-GalNAc(Ac)3)-OH | 133575-43-6 | C33H38N2O13 | Yes&No |
| beta-D-Galactose pentaacetate | 4163-60-4 | C16H22O11 | Yes&No |
| 1,2,3,4,6-PENTA-O-ACETYL-ALPHA-D-GALACTOPYRANOSE | 4163-59-1 | C16H22O11 | Yes&No |
Mannose
Mannose has an important significance and role in protein glycosylation and is involved in many types of glycosylation modifications, including N-glycosylation, O-glycosylation, and C-glycosylation. These glycosylation modifications are essential for proper folding, stability, solubility, and intracellular transport and localization of proteins.
| Name | CAS | Formula | Acetate Groups Removed |
|---|---|---|---|
| Fmoc-L-Ser(ManNAc)-OH | Yes&No | ||
| Fmoc-Thr(ManNAc)-OH | Yes&No | ||
| α-D-MANNOSE ENTAACETATE | 4163-65-9 | C16H22O11 | Yes&No |
| D-MANNOSE PENTAACETATE | 25941-03-1 | C16H22O11 | Yes&No |
| D-Mannopyranose tetraacetate | 140147-37-1 | C14H20O10 | Yes&No |
N-glycosylation starts in the ER, while O-glycosylation primarily occurs in the Golgi apparatus.
The two types of glycosylation are N-glycosylation and O-glycosylation, based on the amino acid attachment sites.
Protein glycosylation is crucial for proper folding, stability, signaling, and cell-cell communication, influencing diverse biological processes.

References