The chemists and biologists of Creative Peptides have extensive experience in synthesizing a variety of post-translationally modified peptides, providing superior post-translationally modified peptides for proteomics and epigenetics, etc. We will make appropriate modifications to the peptide according to our customer's needs.
Post-translational modifications (PTMs) are changes to proteins or peptides that are catalyzed by enzymes after completion of ribosomal translation and generally have a positive impact on peptide activity. PTMs play an important role in virtually all biological processes, therefore, the synthesis of PTM peptides is crucial for many projects.
PTMs alter the physicochemical properties of the peptide by altering the electrostatic charge, hydrophilicity, and conformation, thereby modulating their ability to specifically bind to the protein of interest. Some nascent peptide chains can be directly folded into the final three-dimensional structure after being released from the ribosome, but in most cases, the nascent peptide undergoes a series of processing modifications to function. In general, post-translational modifications are for functional needs, and in another case, the need to fold into a natural conformation.
Post-translational modifications (PTMs) occur in various cellular compartments, with specific modifications being predominant in particular locations. Here are more detailed explanations of where and how these modifications take place:
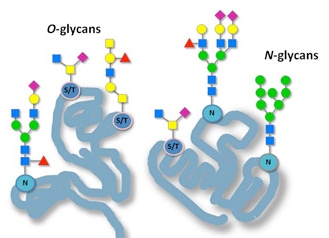
Glycosylation: N-linked glycosylation begins in the ER where oligosaccharides are attached to asparagine residues of nascent polypeptides. This modification is crucial for proper protein folding and stability.
Disulfide Bond Formation: The formation of disulfide bonds between cysteine residues helps stabilize the tertiary and quaternary structure of proteins. This process is facilitated by protein disulfide isomerase (PDI) in the ER.
Folding and Quality Control: Chaperone proteins in the ER, such as BiP, assist in protein folding. Misfolded proteins are targeted for degradation via the ER-associated degradation (ERAD) pathway.
Further Glycosylation: O-linked glycosylation occurs in the Golgi, where sugars are added to serine or threonine residues. Additionally, complex modifications of N-linked oligosaccharides initiated in the ER are further processed in the Golgi.
Proteolytic Processing: Some proteins undergo proteolytic cleavage in the Golgi, which is essential for their activation. Examples include the processing of prohormones and viral glycoproteins.
Sulfation: The addition of sulfate groups to tyrosine residues and carbohydrates can also occur in the Golgi, which is important for protein function and cell signaling.
Phosphorylation: This reversible modification, typically on serine, threonine, or tyrosine residues, is mediated by kinases and reversed by phosphatases. It plays a crucial role in regulating cellular processes such as signal transduction, cell cycle progression, and apoptosis.
Ubiquitination: Proteins are tagged with ubiquitin, a small regulatory protein, which signals for their degradation by the proteasome, alters their cellular location, or affects their activity. This process involves a cascade of enzyme activities (E1, E2, and E3 enzymes).
Sumoylation: Similar to ubiquitination, SUMO (Small Ubiquitin-like Modifier) proteins are attached to lysine residues, influencing protein stability, nuclear-cytosolic transport, and transcriptional regulation.
Methylation: Addition of methyl groups, often on lysine or arginine residues, influences protein-protein interactions and gene expression, particularly in histones.
Acetylation: Typically occurs on lysine residues and affects protein stability and gene expression, with histone acetylation being a key mechanism for regulating chromatin structure and gene transcription.
Phosphorylation: Nuclear proteins, including transcription factors and histones, are often phosphorylated, affecting their activity and interactions.
Methylation: Histone methylation is critical for regulating gene expression by altering chromatin structure and recruiting specific binding proteins.
Acetylation: Histone acetylation, catalyzed by histone acetyltransferases (HATs), is associated with gene activation. Deacetylation by histone deacetylases (HDACs) generally correlates with gene repression.
SUMOylation: Sumoylation of nuclear proteins can regulate their subcellular localization, transcriptional activity, and stability.
N-terminal Acetylation: Many mitochondrial proteins undergo N-terminal acetylation, which can influence their stability and function.
Proteolytic Processing: Mitochondrial targeting sequences are often cleaved upon import into the mitochondria, ensuring proper localization and function of mitochondrial proteins.
Phosphorylation: Some mitochondrial proteins are phosphorylated, which can regulate mitochondrial dynamics, bioenergetics, and apoptosis.
Palmitoylation: The addition of palmitic acid (a lipid) to cysteine residues, which helps anchor proteins to the plasma membrane, thereby influencing their localization and function.
Myristoylation: The addition of myristic acid (a lipid) to the N-terminal glycine residue, playing a role in membrane association and protein-protein interactions.
Glypiation: The addition of glycosylphosphatidylinositol (GPI) anchors, which attach proteins to the extracellular face of the plasma membrane, playing roles in signal transduction and cell adhesion.
N-linked Glycosylation: Continues along the secretory pathway, ensuring proteins are correctly modified before reaching their final destinations.
Proteolytic Cleavage: In secretory vesicles, proproteins are often cleaved to their active forms, such as proinsulin being cleaved to insulin.
Regulation of Protein Activity: PTMs enable precise regulation of protein activity, allowing cells to adapt rapidly to changes in their environment or internal state.
Increased Protein Diversity: PTMs contribute to protein diversity by altering protein functions and interactions, providing a way to generate multiple functional forms from a single gene.
Modulation of Protein Interactions: PTMs can enhance or inhibit protein-protein interactions, influencing cellular signaling pathways and complex formation.
Control of Protein Stability: Modifications such as ubiquitination and acetylation can control protein stability and turnover, ensuring proper protein levels and function.
Localization and Targeting: PTMs like lipidation and glycosylation affect protein localization within the cell, guiding proteins to specific cellular compartments or membranes.
Regulation of Cellular Processes: By modulating enzyme activity, protein folding, and gene expression, PTMs play crucial roles in regulating cellular processes such as metabolism, cell cycle, and apoptosis.
Response to External Stimuli: PTMs allow proteins to respond dynamically to external stimuli such as stress, hormones, or environmental changes, facilitating cellular adaptation and survival.
Control of Cellular Signaling: PTMs such as phosphorylation and acetylation are key regulators of signal transduction pathways, affecting cellular responses to signaling molecules.
Enhancement of Protein Function: Modifications can enhance or refine protein functions, including enzymatic activity, molecular recognition, and structural stability.
Cellular Identity and Communication: Glycosylation and other modifications contribute to cell-cell communication and recognition, impacting immune responses and tissue formation.
Drug Development: PTMs are targeted to design drugs that can specifically modulate the activity or stability of proteins involved in disease, such as protein kinase inhibitors or monoclonal antibodies.
Biomarker Discovery: Changes in PTMs can serve as biomarkers for disease diagnosis, progression, or prognosis, helping to identify new therapeutic targets and monitor treatment responses.
Protein Engineering: PTMs are used to modify proteins for industrial and therapeutic applications, such as enhancing enzyme stability or optimizing antibody efficacy.
Cancer Research: Studying PTMs in cancer cells helps understand tumorigenesis and identify novel targets for cancer therapy, as many cancer-related proteins undergo abnormal modifications.
Neuroscience: PTMs are critical in studying neurodegenerative diseases and brain function, as alterations in protein phosphorylation, acetylation, and ubiquitination can affect neural processes and disease mechanisms.
Vaccine Development: PTMs are used to design vaccines with enhanced immunogenicity, such as glycosylated antigens that improve immune responses.
Cell Signaling Studies: Analyzing PTMs helps elucidate cellular signaling pathways and their regulation, providing insights into how cells communicate and respond to external signals.
Diagnostic Tools: PTM-specific antibodies and assays are used in diagnostics to detect and quantify modified proteins, aiding in the detection of diseases and monitoring of physiological states.
Biotechnology: PTMs are leveraged in biotechnological processes to improve the yield and functionality of recombinant proteins, such as optimizing glycosylation patterns for better therapeutic efficacy.
Structural Biology: Understanding PTMs contributes to elucidating the structure-function relationship of proteins, as modifications can influence protein folding, stability, and interactions.
If you need a post-modification not listed below, or have any questions about peptide modifications, please feel free to contact us. You will receive a reply within 24 hours.
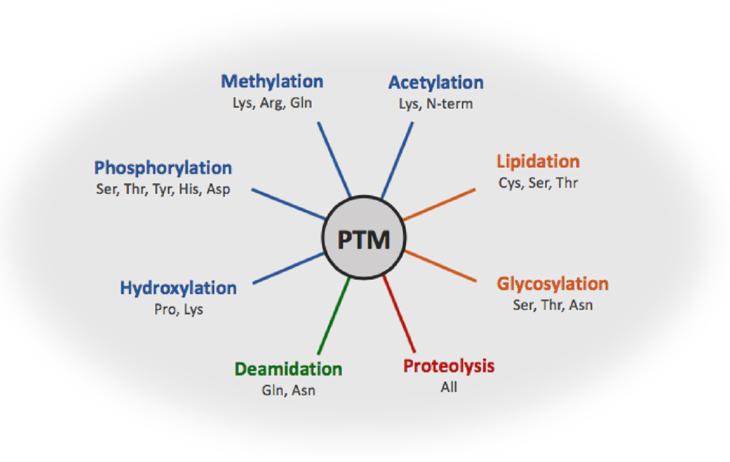
Phosphorylation: Phosphorylation of a polypeptide refers to a form in which a Ser, Thr or Tyr side chain hydroxyl group in a peptide chain is converted to a phosphate by enzymatic or chemical methods. Phosphate is negatively electroned, when a polypeptide or protein is modified by a phosphate, its conformation will change greatly, affecting the interaction between the enzyme and the substrate. Many physiological processes mediated by peptides and proteins are achieved by the addition and removal of phosphate on the substrate.
Glycosylation: Glycopeptides, especially the glycan chains, have played pivotal roles in various biological activities and involved in numerous biological recognition events, such as immune system, endocrine system, protein folding and cell communication. Synthetic glycopeptides have provided a unique frontier for the investigation and better understanding in both glycobiology and proteomics, and also contribute to the development of either biotechnological or therapeutic applications.
Acetylation: N-terminal acetylation can reduce the overall charge of the polypeptide, but solubility may decrease. Since the stability of the polypeptide can be increased, the terminal block is made closer to the parent protein, and thus such modification has the potential to enhance the biological activity of the polypeptide.
Sulfation: Sulfurylation on Ser, Thr and Tyr in polypeptides is a common modification. The activity of many enzymes depends on the oxidation state of the SH groups in these residues. We establish a reliable peptide sulfonation program that guarantees a high success rate.
Hydroxylation: Hydroxyproline and hydroxylysine are hydroxylation methods in polypeptide modification. We can provide hydroxylation of proline and lysine sites in peptides to meet customer research needs.
Prenylation: In eukaryotic cells, a specific set of proteins are modified by a C-terminal plus 15 carbon farnesyl group or a 20-carbon geranylgeranyl isoprenoid moiety called prenylation. We can also add these two groups to the peptide to prenylate them at the request of our customers.
 (Forrest S, et al., 2020)
(Forrest S, et al., 2020)
Comprehensive Modification Options: We offer a wide array of modifications, including phosphorylation, glycosylation, ubiquitination, acetylation, and methylation, to meet diverse research needs.
High Precision and Specificity: Our advanced technology and specialized protocols ensure precise and specific modifications for reliable experimental outcomes.
Expert Consultation and Support: Our experienced scientists provide expert guidance and support at every step of your post-translational modification experiments.
Advanced Analytical Techniques: We use cutting-edge techniques like mass spectrometry and HPLC to verify and characterize modifications with high accuracy.
Customizable Services: We offer highly customizable services to fit the specific requirements of your research or industrial applications.
Rapid Turnaround Times: Our efficient workflows enable rapid turnaround times without compromising on quality, accelerating your project timelines.
Scalability: We can scale our services to accommodate both small-scale research projects and large-scale industrial applications.
Quality Assurance: We adhere to stringent quality control measures to ensure the highest standards of purity and functionality in every modified protein.
Post-translational modifications (PTMs) are chemical modifications made to a peptide or protein after its synthesis, which are essential for regulating its activity, stability, and interaction with other molecules. These modifications play a key role in biological processes like protein folding, signaling, and enzyme activity.
PTMs can alter the peptide's physicochemical properties, such as charge, hydrophobicity, and conformation. These changes can enhance or inhibit the peptide's ability to interact with target proteins or other biomolecules, thereby modulating its biological activity and specificity.
PTMs occur in various cellular compartments. For example, glycosylation happens in the endoplasmic reticulum (ER) and Golgi apparatus, phosphorylation typically occurs in the cytoplasm or nucleus, and acetylation often takes place on histones in the nucleus, influencing gene expression.
We offer a wide range of PTM services, including phosphorylation, glycosylation, acetylation, sulfation, hydroxylation, and prenylation. Each of these modifications can be performed enzymatically or chemically to meet specific research needs.
PTMs are crucial in protein engineering as they can enhance protein function, stability, and specificity. By introducing modifications like phosphorylation or acetylation, peptides can be engineered for better performance in various applications, such as proteomics, biomarker discovery, and structural studies.
Yes, we specialize in synthesizing peptides with multiple PTMs, providing flexibility in designing peptides with enhanced properties for specific research applications, such as improved binding affinity or better solubility.

References