Surface plasmon resonance imaging (SPRi) represents a paradigm shift in the study of biomolecular interactions, offering real-time, label-free detection with high sensitivity and spatial resolution. As a leading provider of SPRi services, Creative Peptides is committed to empowering researchers and innovators with state-of-the-art tools to unravel the mysteries of life at the molecular level.
SPRi, namely surface plasmon resonance microscopy (SPRM), is a real-time, label-free, and high-throughput technique which is used to study biomolecular interactions based on detecting the refractive index changes resulting from molecular binding. This technology is built upon the principle of surface plasmon resonance (SPR), which occurs when polarized light hits a metal surface under certain conditions, causing the electrons in the metal to resonate. This resonance is highly sensitive to changes in the refractive index of the medium surrounding the metal surface. By monitoring changes in the resonance condition, induced by molecular binding events on the sensor surface, SPRi provides real-time, quantitative information about biomolecular interactions, including kinetics, affinity, and concentration.
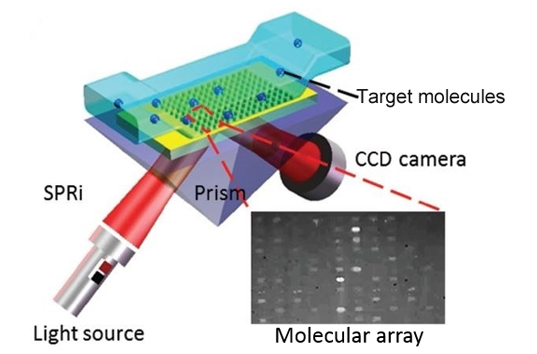
While both SPR and SPRi harness the phenomenon of surface plasmon resonance for studying molecular interactions, they differ in their detection methods. Traditional SPR relies on a single sensing spot, limiting its ability to provide spatial information about biomolecular interactions.
SPRi extends the capabilities of traditional SPR by allowing for the simultaneous imaging of multiple interactions in real-time. Instead of measuring a single point on the sensor surface, SPRi systems utilize imaging sensors to capture the SPR signal across the entire surface. In SPRi, the sensor surface is typically divided into an array of discrete sensing regions, each functionalized with a different capture molecule. When analyte solutions are flowed over the surface, binding events at each sensing region produce localized changes in the SPR signal, which are captured by the imaging system. By analyzing the spatial distribution of these changes, researchers can obtain multiplexed binding data for multiple interactions simultaneously. SPRi offers the additional capability of spatially resolving and imaging biomolecular interactions across a sensor surface, making it a powerful tool for high-throughput screening and multiplexed analysis.
1. Preparation of a gold-coated substrate: A thin layer of gold is deposited onto a glass slide or coverslip.
2. Immobilization of ligands: Biomolecules (ligands) of interest are immobilized onto the gold surface.
3. Sample injection: The sample containing the analyte (molecules to be studied) is flowed over the surface.
4. Imaging: Polarized light is directed onto the gold surface at a specific angle, and changes in SPR are visualized and recorded using a microscope. This allows for the real-time monitoring of molecular interactions and binding kinetics.
SPRi offers a valuable tool for studying bacterial biofilms due to its ability to provide real-time, label-free, and spatially resolved information about biomolecular interactions on a surface. Here's how SPRi can be used in the study of bacterial biofilms:
Characterization of biofilm formation: SPRi can be employed to monitor the formation of bacterial biofilms in real-time. By immobilizing relevant bacterial adhesion molecules or extracellular polymeric substances (EPS) onto the sensor surface, researchers can observe the attachment of bacteria and the subsequent growth of the biofilm over time. This allows for the investigation of biofilm initiation, growth kinetics, and structural development.
Analysis of biofilm interactions: SPRi enables the study of interactions between bacterial cells and various substrates or biomolecules. By functionalizing the sensor surface with different molecules (e.g., proteins, carbohydrates, peptides), researchers can investigate the adhesion mechanisms of bacteria to specific ligands. This can include studying the role of specific adhesins, surface receptors, or extracellular matrix components in mediating biofilm formation and stability.
Screening of antibiofilm agents: SPRi can be used to screen and evaluate potential antibiofilm agents or inhibitors. By exposing pre-formed biofilms to candidate compounds or antimicrobial agents, researchers can monitor changes in biofilm integrity, biomass, or architecture in real-time. This allows for the rapid assessment of the efficacy of antibiofilm treatments and the identification of promising therapeutic candidates.
Mapping biofilm heterogeneity: SPRi provides spatially resolved imaging of biofilm interactions, allowing researchers to map the distribution of bacterial cells, EPS, and other biofilm components across the sensor surface. This spatial information can reveal insights into biofilm heterogeneity, such as the presence of microcolonies, spatial gradients in bacterial density or metabolic activity, and the localization of matrix components.
Investigation of biofilm dynamics: SPRi can be used to study dynamic processes within biofilms, such as bacterial motility, quorum sensing, and matrix production. By monitoring changes in SPR signals over time and in response to environmental stimuli (e.g., nutrient availability, pH, temperature), researchers can gain insights into the spatiotemporal dynamics of biofilm formation, growth, and dispersal.
Kinetic characterization by Surface Plasmon Resonance and other methods has already been an important step for drug researchers to select and characterize novel therapeutics as well as for basic life scientists to investigate specific biological binding events. When combined with appropriate surface chemistry, microfluidics and software, SPRi is unmatched in its range of applications including:
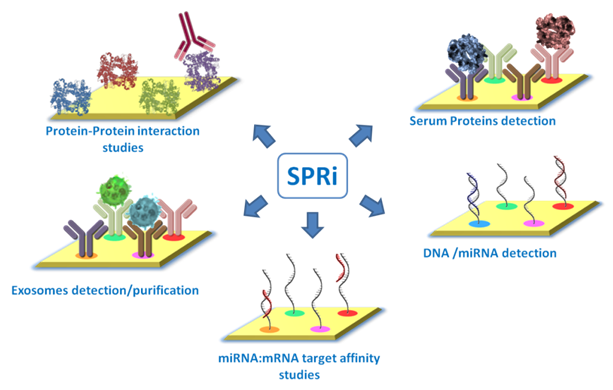
In conclusion, SPRi is a well-established leading technology for measuring binding association (ka) and dissociation rates (kd), affinities (KD). Creative Peptides offers SPRi (Surface Plasmon Resonance imaging) services including Biochip design and printing, Bio-interactions analysis (binding affinity and kinetic processes detection), Summary and analysis of the results. Typical SPRi services include:
As for our clients, all you need to do is just tell us the essential details of the experiment, and then our scientists will provide a comprehensive solution to match their scenario. At the end of the service, they will get the result of the SPRi and the full experiment data.
SPRi relies on the principle of surface plasmon resonance, where changes in refractive index on a sensor surface, due to molecular binding events, alter the resonance condition of incident light. This change is detected as a shift in the SPR angle, providing quantitative information about binding kinetics and affinity.
SPRi can be used to investigate a wide range of interactions, including protein-protein, protein-DNA/RNA, protein-small molecule, antibody-antigen, and receptor-ligand interactions.
SPRi offers several advantages, including label-free detection, real-time monitoring of interactions, high sensitivity, minimal sample consumption, and the ability to study complex biological samples such as serum, cell lysates, and crude extracts.
We can analyze various types of samples, including purified proteins, antibodies, nucleic acids, small molecules, peptides, and complex biological samples.
Our SPRi data analysis provides comprehensive information, including kinetic parameters (association rate constant, dissociation rate constant, equilibrium dissociation constant), binding affinity, stoichiometry, and thermodynamic parameters (enthalpy, entropy).
The turnaround time for SPRi analysis depends on the complexity of the project and sample availability. We strive to deliver results in a timely manner and can discuss specific timelines based on your requirements.
Yes, our team of experts can assist you with experimental design, optimization of assay conditions, data interpretation, and recommendations for further experiments based on the results obtained from SPRi analysis.
Yes, our SPRi platform is capable of handling high-throughput screening projects, enabling rapid screening of compound libraries or protein interactions.

References