Creative Peptides specializes in the commercial production of peptides active pharmaceutical ingredients (APIs), cosmetic peptidesand functional peptides, as well as the development of custom peptide manufacturing processes. We are dedicated to providing proprietary and generic cGMP-grade peptides to pharmaceutical companies and the biotechnology market.
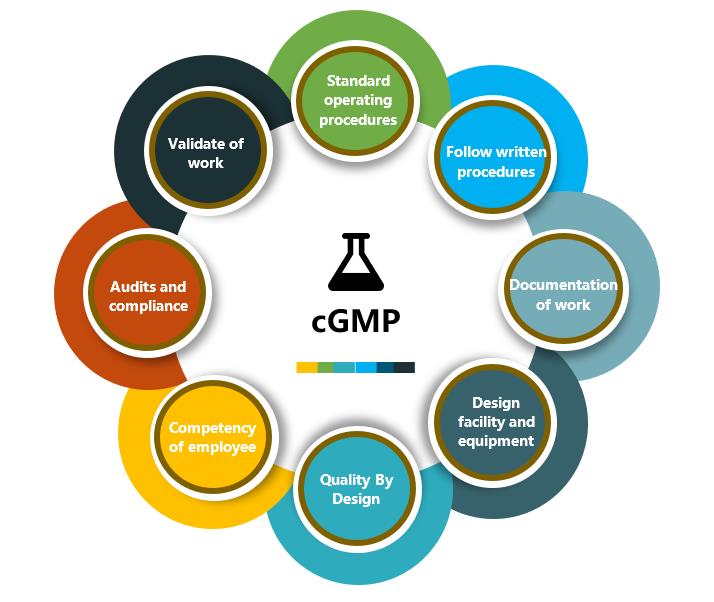
cGMP is the regulation provided by the FDA that guides the design, monitoring, and maintenance of manufacturing facilities and processes. Creative Peptides adheres to cGMP guidelines and has FDA-inspected manufacturing facilities to ensure superior quality of the peptides produced. Our team of peptide experts has the experience and expertise to implement cGMP services throughout the entire project process to help accelerate your peptide drug development process for eventual commercialization.
With the continuous progress of peptide development technology, such as chemical synthesis, fermentation, phage display, and computer-aided drug design (CADD), the structure of peptide molecules has changed (e.g. the average length of peptide sequences is gradually increasing), and the combination forms of peptide molecules are also more diversified. Initially, peptide drugs consisted of natural amino acids, and then D-amino acids or other unnatural amino acids were introduced, and gradually expanded to fatty acid modification, PEGylation, as well as coupling with proteins, small molecules, or other functional groups.
Creative Peptides has established a strict quality management system, using the latest GMP compliant technologies and systems, cGMP compliant facilities and a highly qualified quality management team. We are committed to providing high quality peptide products and services to our customers. At present, we have successfully completed several cGMP peptide APIs production projects.
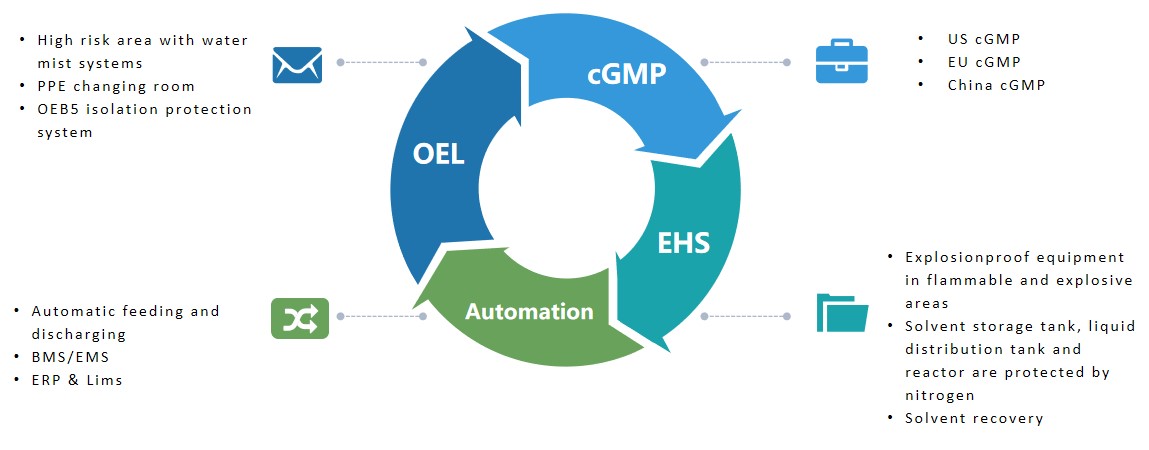
Creative Peptides' cGMP manufacturing site is designed in strict compliance with FDA guidelines, enabling large-scale cGMP production of peptides. As a leading global peptide manufacturer, our team has the experience and expertise to enable drug discovery and development support.
cGMP ensures that peptides are produced in compliance with strict quality control measures, maintaining the highest standards for purity and consistency, which is critical for research and industrial applications.
We implement a rigorous quality management system that includes electronic batch record auditing, analytical testing, and corrective actions to guarantee the safety, strength, and purity of each peptide batch.
Yes, Creative Peptides specializes in custom peptide synthesis, including modifications such as backbone alterations and the introduction of non-natural amino acids or functional groups, to meet your exact research needs.
We offer a wide range of peptide modifications, including Boc-, Fmoc-, and Cbz- protect groups, as well as fatty acid modifications, PEGylation, and peptide coupling with proteins or small molecules.
Our facilities are equipped to handle both small-scale and large-scale peptide synthesis, ranging from grams to multi-kilogram quantities, making it possible to scale production as your needs evolve.
We employ advanced technologies such as chemical synthesis, fermentation, and computer-aided drug design (CADD) to ensure the production of high-quality peptides with diverse structures and functionalities.
We provide comprehensive peptide analysis services, including purity testing, structural verification, and potency assessments, to ensure that your peptides meet the required specifications.
Using cGMP-grade peptides guarantees that the peptides meet industry standards for quality, consistency, and reproducibility, ensuring reliable results for research, development, and manufacturing.
