| CAT# | Product Name | M.W | Molecular Formula | Inquiry |
|---|---|---|---|---|
| A12001 | Amylin(20-29), Islet Amyloid Polypeptide, IAPP (20-29), rat | 1007.2 | ||
| A12002 | Amylin (20-29), human | 1009.1 | ||
| A12003 | Amylin(20-29), Islet Amyloid Polypeptide, IAPP (20-29), cat | 1019.1 | ||
| A12004 | IAPP (20-29), hamster | 1024.2 | ||
| A12006 | Amylin (1-13), human | 1378.6 | ||
| A12009 | Amylin (8-37), human | 3183.5 | ||
| A12011 | Acetyl-Amylin (8-37) (human) | 3225.53 | C140H218N42O46 | |
| A12012 | Acetyl-Amylin (8-37), rat | 3243.7 | ||
| A12013 | Amylin (1-37), human, amide | 3903.3 | ||
| A12014 | Amylin, human, free acid | 3904.3 | C165H258N50O55S2 | |
| A12015 | Amylin (1-37), human | 3906.3 | ||
| A12016 | Amylin, rat | 3920.5 | C167H272N52O53S2 | |
| A12017 | Pramlintide, Acetate [Pro25, 28, 29]-Amylin(1-37) (human), Amide | 3949.6 | C171H269N51O53S2 | |
| A12018 | 5-FAM-Amylin (mouse, rat) | 4278.75 | C188H282N52O59S2 | |
| A12019 | Amylin (14-20) (human) | 802.89 | C36H54N10O11 | |
| A12020 | Amylin (20-29) (human) | 1009.1 | C43H68N12O16 | |
| CAD-108 | Biotinyl-Amylin (human) | 4129.63 | C₁₇₅H₂₇₅N₅₃O₅₇S₃ | |
| CAD-109 | 5-FAM-Amylin (human) | 4261.64 | C₁₈₆H₂₇₁N₅₁O₆₁S₂ |
Amylin, which is also known as islet amyloid polypeptide (IAPP), is a peptide of 37 amino acids. It is normally produced and co-expressed with insulin in β-cells and other kinds of cells at a ratio ranging from 1:10 to 1:100. The 89-amino acid PrePro-peptide is the starting point for IAPP expression. In the endoplasmic reticulum (ER), peptidases break it to the Pro-peptide. In the Golgi apparatus, prohormone convertase 2, prohormone convertase 1/3, and carboxypeptidase E produce the mature IAPP. For IAPP to exert its maximum biological action, amidation of the C-terminal glycine-residue is also necessary. Interaction between IAPP and calcitonin gene-related peptide (CGRP) is characterized by very similar amino acid sequences. Being a member of the calcitonin peptide family, IAPP follows suit. There is currently no proof that there is a receptor that is unique to amylin. In the brains of rats and mice, however, there have been reports of calcitonin receptors that are heterodimerized with RAMPs. These proteins exhibit an affinity for IAPP.
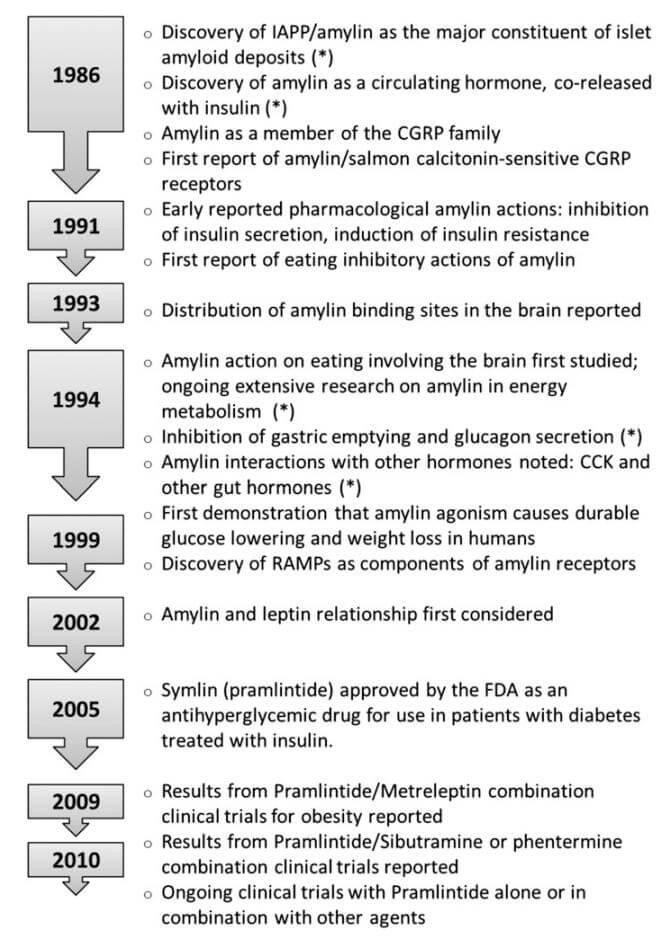
A timeline of major discoveries and achievements in the field of amylin biology. (Hay D L., et al., 2015)
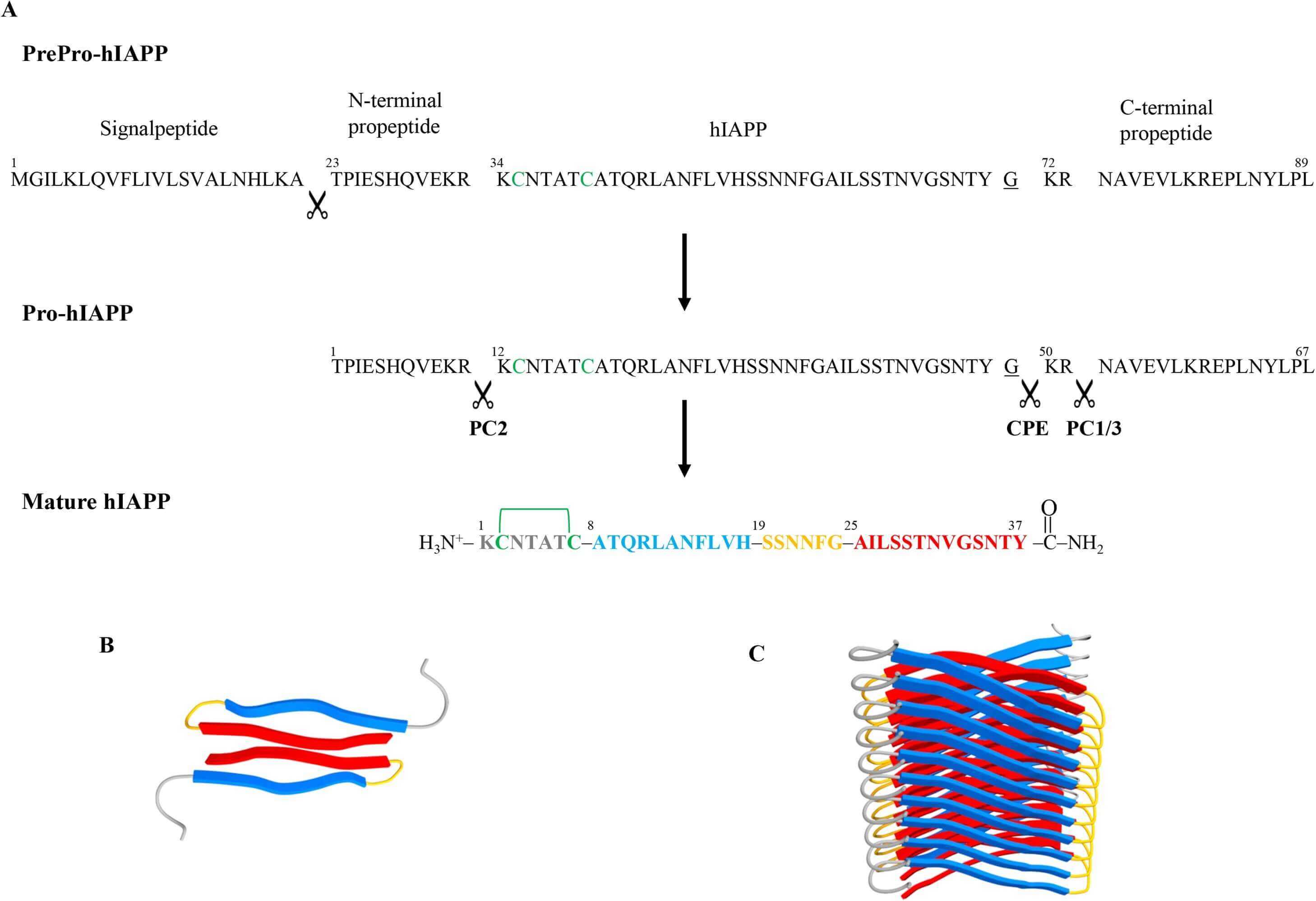
Amino acid sequences and processing steps leading to mature hIAPP (Amylin). (Press M., et al., 2019)
When combined with insulin and leptin, amylin has a direct effect on hunger. Amylin makes the stomach work more slowly to evacuate its contents. Amylin inhibits pancreatic secretion of glucagon after a meal. When we eat less, it's because amylin has activated a number of reward and homeostatic areas in our brains. Animal studies imply that amylin may affect the efferent activity of the vagus nerve via neurons in the brainstem, although the exact method by which amylin delays gastric emptying is yet unknown. An open blood-brain barrier and a dense population of amylin receptors define the region postrema (AP) of the brainstem. Class B calcitonin G protein-coupled receptors (CTRs) and receptor-activity modifying proteins (RAMPs) form heterodimers known as amylin receptors. There are three separate amylin receptors that interact with different types of RAMPs: AMY1, AMY2, and AMY3. By altering receptor specificity and affinity, RAMPs transform the pharmacology of calcitonin receptors from those that prefer calcitonin to those that prefer amylin. When amylin-activated neurons bind in the region postrema, they send a projection to the lateral parabrachial nucleus. There, glutaminergic neurotransmission explains how amylin makes you feel full. From what we can tell, the AP limits glucagon secretion and slows stomach emptying, which are mechanisms by which amylin exerts its satiating effect. Future research should focus on clarifying the nature of the connections between these neurons, particularly the lateral parabrachial nucleus pathway. The absence of co-receptors for GLP-1 and GLP-15 in amylin-responsive neurons in the AP is significant since it indicates that these peptides target distinct principal cells. Although there is some evidence that GLP-1 and CTR receptors overlap in the nucleus tractus solitarius, additional research is needed to understand how neurons triggered by GLP-1 and amylin interact with one another.
The 37-amino acid hormone amylin originates from an 89-amino acid precursor and goes by many names, including islet/insulinoma amyloid polypeptide and diabetes-associated peptide. Amylin is produced from prohormones by means of prohormone convertases 1/3 and 2, as well as carboxy peptidase E. This peptide's encoding gene is located on human chromosome 12 (12p12.1). To achieve maximum bioactivity, two significant post-translational changes are necessary: a C-terminal amide (Tyr37) and the formation of a disulfide link between the two cysteine residues at positions 2 and 7. The amino acid sequence of amylin shows the most similarity to calcitonin gene-related peptide (CGRP). comparable to CGRP, it has an amidated C terminus and a disulfide bond at a comparable location. Adrenomedullin 2, calcitonin, and adrenomedullin all have this property. certain peptides have certain traits, which bring them together to create a tiny family. Because of this, the corresponding receptors for the various peptides and their pharmacological effects are quite similar.
Amino acid sequence and pharmacology similarities and variations between these peptides and across species have been used to create agonists and antagonists that imitate or inhibit amylin action. One of the most prominent is pramlintide, a synthetic analogue of human amylin that is both bioactive and reasonably stable. A powerful amylinomimetic agent, pramlintide is on par with human amylin. Like human amylin, it is a 37-amino-acid polypeptide; however, it varies in amino acid sequence from human amylin due to the substitution of proline for alanine at positions 25, 28, and 29. As the bioactive peptide analog of amylin, pramlintide varies from it by three amino acids but is otherwise stable. Patients with type I diabetes mellitus had lower postprandial glucose concentrations when administered pramlintide doses that are linked to amylin levels within the physiological range.
AM833 (cagrilintide), a unique experimental long-acting acylated amylin analogue, functions as a non-selective amylin receptor agonist. This calcitonin G protein-coupled receptor agonist presents a promising therapeutic therapy for obesity, leading to decreased food consumption and substantial weight loss in a dose-dependent fashion.
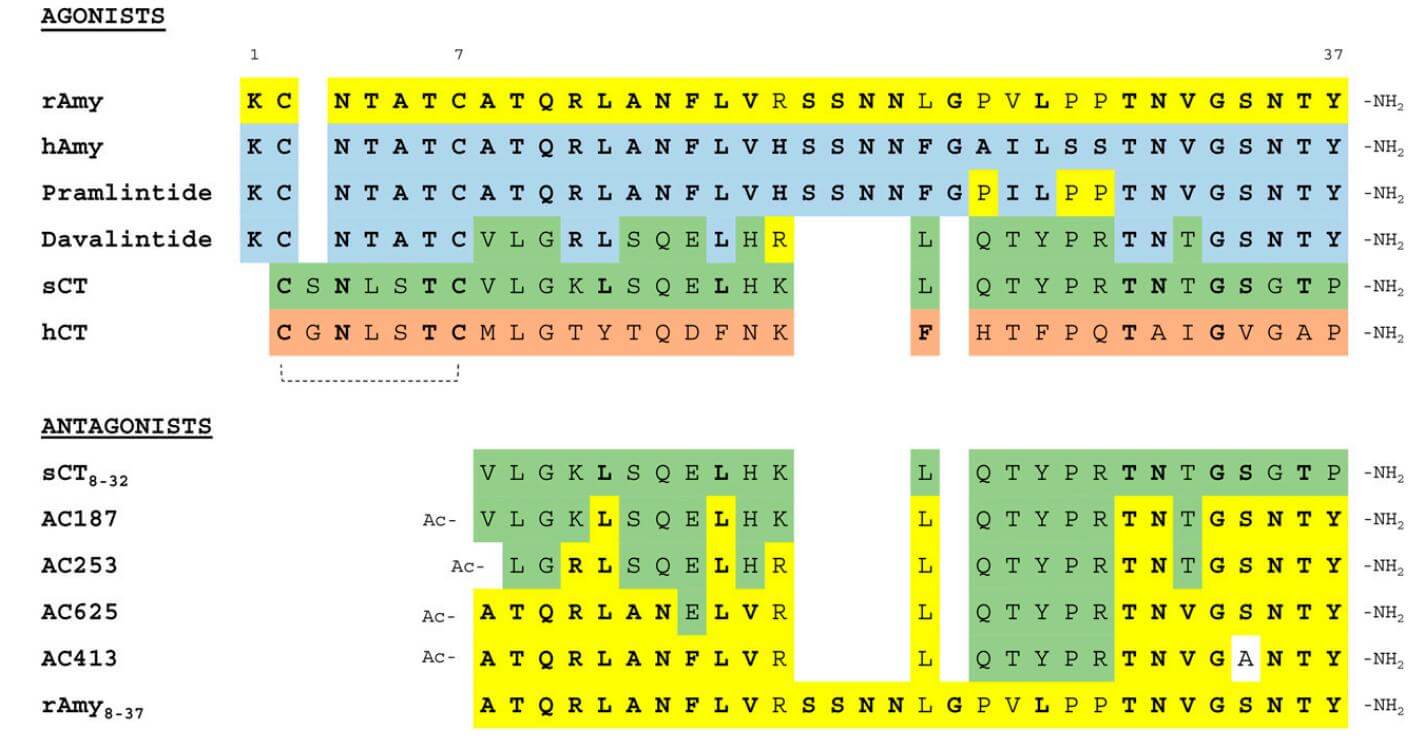
Amino acid sequences of amylin and related peptides. (Hay D L., et al., 2015)
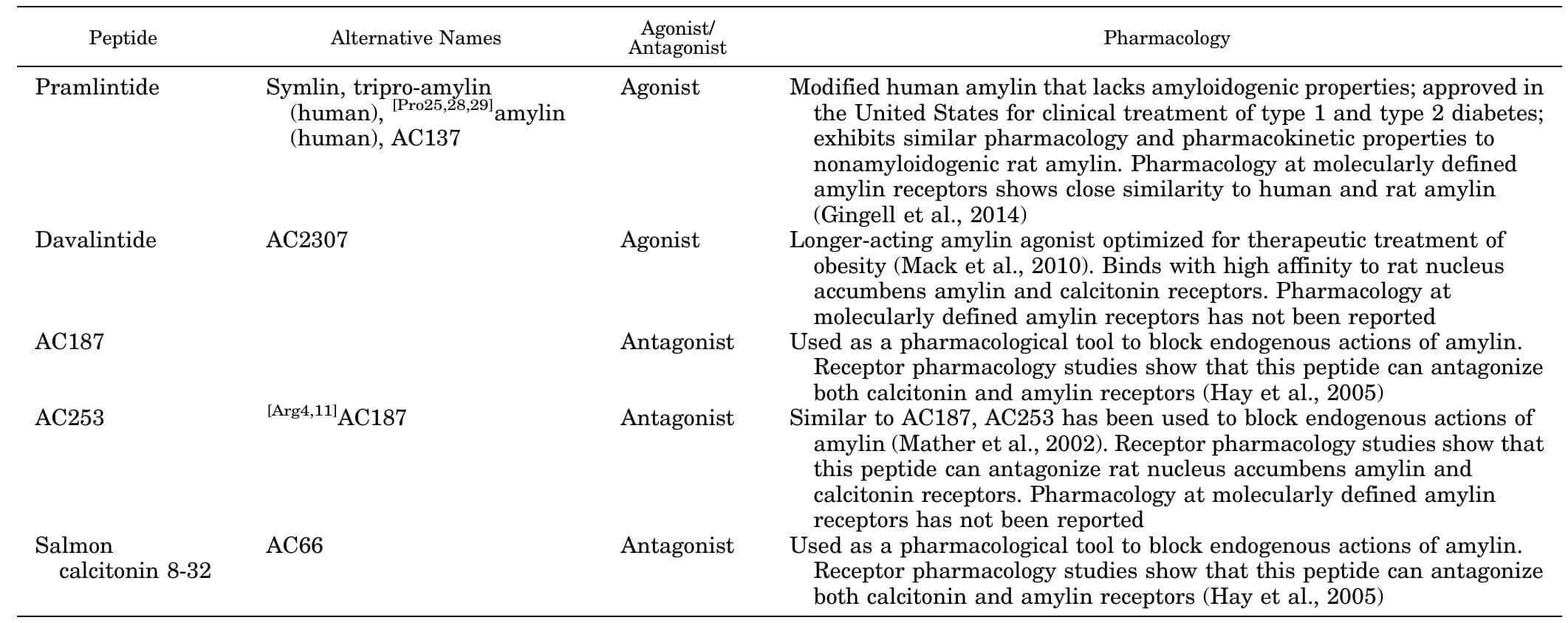
Summary of clinically relevant or commonly used amylin-related peptides and their effects. (Hay D L., et al., 2015)
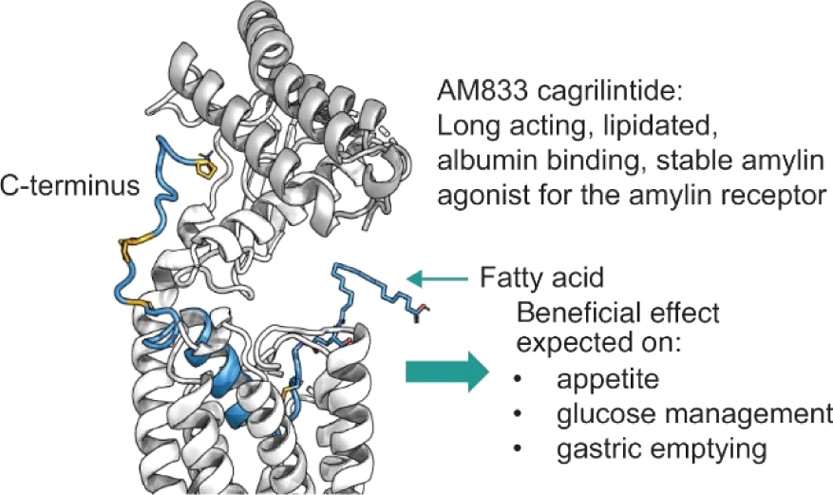
Cagrilintide (AM833) is the Amylin analog. (Kruse T., et al., 2021)
Pramlintide controls blood sugar levels after eating by reducing the rate of stomach emptying, increasing feelings of fullness, and preventing diabetes-related abnormalities in the increase of glucagon after a meal. Because of this improvement in regulation, exogenous insulin treatment may more readily adapt to physiological demands by regulating both endogenous (derived from the liver) and exogenous (derived from meals) glucose input. These effects are counteracted when blood glucose levels decrease, as they are glucose dependant. When no additional medications are present that can trigger hypoglycemia, pramlintide has no effect on blood sugar levels. In healthy individuals, pramlintide at supraphysiologic levels does not cause hypoglycemia, and it does not impede the recovery process after hypoglycemia caused by insulin.
Cagrilintide, formerly known as NNC0174-0833, AM833, is a long-acting amylin analogue that continues to pique researchers' interest due to its likely additive effect on appetite reduction when combined with semaglutide. Similar to amylin, a naturally occurring hormone secreted by pancreatic B cells in reaction to food availability, cagrilintide suppresses hunger by acting on the areas of the brain that are involved in the homeostatic and hedonistic mechanisms of satisfaction. On top of that, it lessens glucagon output and slows down stomach emptying. Patients in a one-center, placebo-controlled, multiple-ascending-dose phase 1b trial were given cagrilintide subcutaneously at a dose of 0.16, 0.3, 0.6, 1.2, 2.4, and 4.5 mg once weekly, or 2.4 mg of semaglutide and a placebo once weekly. Compared to the semaglutide with placebo group, the cagrilintide with semaglutide group showed significantly larger weight loss after 20 weeks, with rates of 15.7% for 1.2 mg and 17.1% for 2.4 mg of cagrilintide, respectively, compared to just 9.8% for the semaglutide alone. Headache, nasopharyngitis, gastrointestinal symptoms (vomiting, diarrhea, constipation, nausea), and responses at injection sites were the most frequently reported adverse events.
Pancreatic islets of diabetics and brains of Alzheimer's sufferers both contain amyloid, which shares many biophysical and physiological characteristics with amylin. Both amyloid-beta (Aβ) and human amylin combine and produce amyloid fibrils, even though they do not share any sequence homology. All three go through the same crucial steps in this process: sequence-specific aggregation, which involves oligomeric intermediates before reaching the aggregated state and the physical state of inclusion bodies. The amyloid fibrils usually do not branch out, have different lengths, can take on different forms, and assemble into crossed β-sheets. It is possible that protein oligomers, fibrils, and monomers are involved in the dynamic process of amyloid plaque development. As a means of storing peptide hormones within secretory granules, amyloid fibrils can play a crucial role in regular cellular functioning. Despite the potential physiological and pathological functions of Aβ or amylin insoluble fibrils, the most harmful form of amyloids is thought to be the soluble oligomeric intermediates. A shared conformation-dependent structure is displayed by numerous soluble oligomers of various proteins, including Aβ, amylin, prion protein, and alpha-synuclein, irrespective of their sequence. It appears that various soluble amyloid oligomers share a common structure and a common mechanism of toxicity, as oligomer-specific antibodies can suppress the oligomer toxicity. The neurotoxicity profiles of Aβ and human amylin are remarkably comparable, which is rather intriguing. The effects of Aβ and amylin on neurotoxicity, potassium channel function, and pro-apoptotic gene activation can be reduced or prevented by using AC187 or AC253, which are antagonists of the amylin receptor. The toxicity caused by oligomerized Aβ is reduced when the expression of the amylin receptor gene is downregulated by siRNA. In addition, certain areas of the brain showed an elevated amyloid burden and an age-dependent upregulation of amylin receptor expression in the TgCRND8 transgenic model mice for Alzheimer's disease, which overexpress APP. In the same way that human amylin can directly activate AMY3, Aβ can also elevate intracellular calcium, cyclic adenosine monophosphate (cAMP), and activation of PKA and MAPK. In addition, AC253 can restore long-term potentiation (LTP) in the hippocampus of AD mice (TgCRND8) by acting on AMY3 receptors. Therefore, it seems that the amylin receptor is the primary means by which Aβ exerts its effects on cells, and these findings provide credence to the idea that oligomer Aβ interacts directly with amylin receptors.
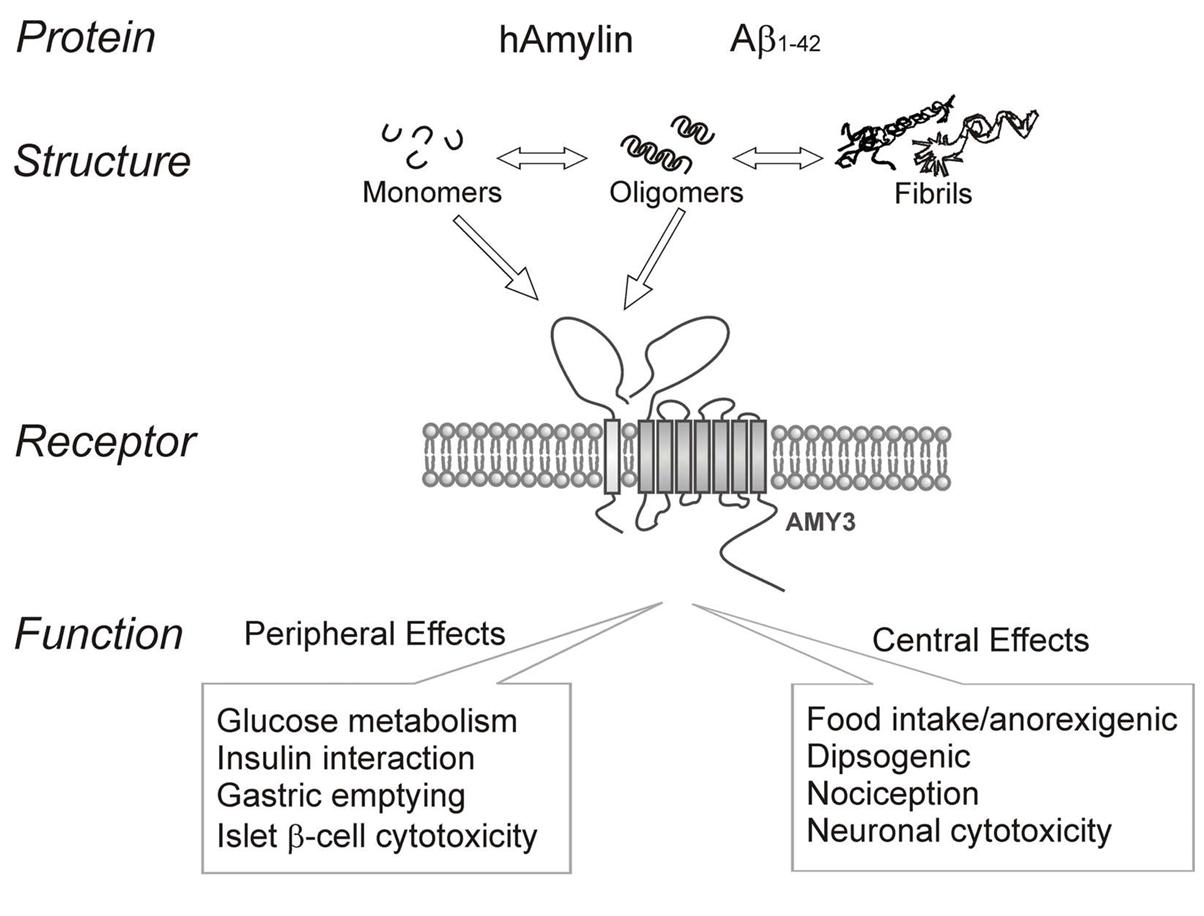
Summary of human amylin and amyloid-beta (Aβ1-142). (Fu W., et al., 2013)
The movement of glucose from the circulation into peripheral tissues like skeletal muscle and the absorption of glucose from digested carbs both contribute to the regulation of blood glucose levels. To facilitate transfer from the blood stream into tissues that rely on insulin, insulin is widely acknowledged to have a role. By reducing the rate of stomach emptying, amylin helps regulate the second component, which is the absorption of glucose into the bloodstream. As a result, Amylin might mediate centrally through receptor-dependent direct brain activity. Since Amylin binding sites are located in the stomach fundus of rats and Amylin mRNA expression is observed in the gastrointestinal tract, it is possible that Amylin directly mediates the suppression of gastric emptying in the gut. Amylin has a calming impact on the ileal smooth muscles of rats, according to one study.
Amylin regulates energy homeostasis by regulating food intake and body weight through the central nervous system-mediated satiation signal. As an obesity signal, it regulates energy expenditure effectively as well. Research has shown that amylin can boost the effects of other hormones like leptin, insulin, and peptide YY in this setting. When administered in conjunction with amylin, leptin has an even greater effect on reducing food intake and body weight. Amylin has the opposite effect on insulin and glucagon secretion. Amylin appears to reduce insulin secretion, according to some investigations; however, other studies failed to find this impact. There have been reports that suggest a reduced conversion of glycogen to glucose due to a restricted production of glucagon from islet α-cells.
It seems that amylin has extra impacts on the endocrine system in the body. It is believed to affect bone metabolism by blocking osteoclast activity and promoting osteoblast growth and activity, as a result of its sequence similarity to CGRP. Beyond its vasodilatory effects, it appears that IAPP is involved in the renin-angiotensin-system. Nevertheless, additional research is needed to completely understand the physiological functions of amylin.
The majority of amylin aggregations form outside of cells. However, β-cells also had modest amounts within their own cells. Despite the publication of multiple theories about the cytotoxic mechanisms of human amylin on β-cells, the cellular processes outlined in these proposals remain incompletely understood. A variety of harmful effects, including membrane rupture, ROS formation, apoptosis induction, impacts on the inflammasome, amyotrophic lateral sclerosis (ALS), and the unidirectional current system (UPS), and processes through endoplasmic reticulum stress (ER stress), are proposed. Because they are believed to be more poisonous than amorphous aggregates, IAPP oligomers have recently attracted a lot of attention from researchers.
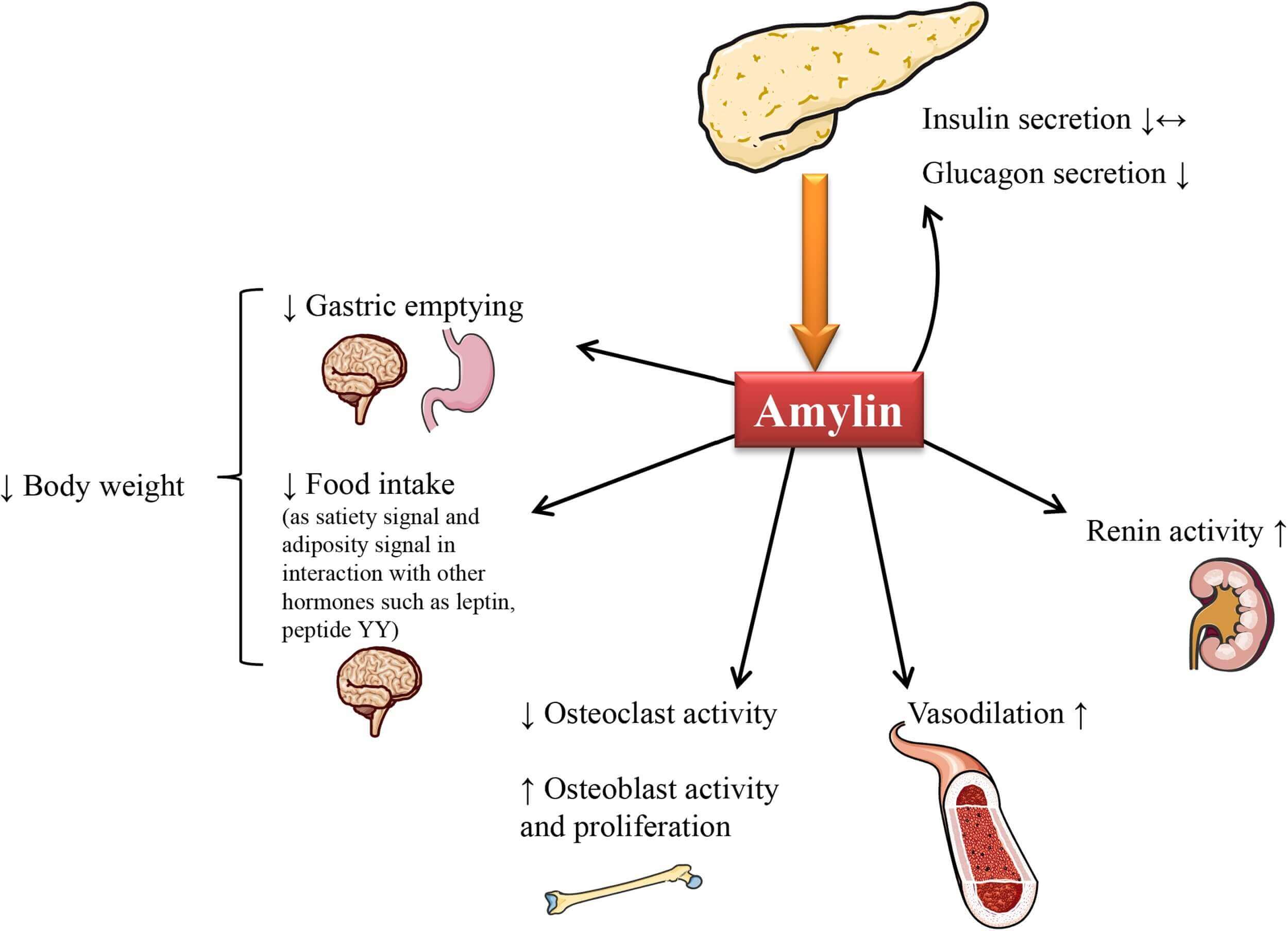
Physiological role of amylin. (Press M., et al., 2019)
Metabolic disorders such as insulin resistance and type 2 diabetes cause heart failure in a variety of ways. Hyperinsulinemia occurs when pancreatic β-cells secrete more insulin to make up for insulin resistance. The hormone amylin, which has a molecular weight of around 4 kDa, is produced by pancreatic β-cells and is secreted with insulin. Previous research demonstrated that amylin decreases insulin secretion and sensitivity while simultaneously modulating eating behavior. On the flip side, amylin may have a function in energy management as removing the gene from mice improved their glucose tolerance. When human amylin is overexpressed, it clumps and forms amyloid. Pancreatic oxidative damage, inflammation, and cell death were all demonstrated to be caused by aggregated amylin. On the other hand, amylin derived from animals that don't naturally have type 2 diabetes (such rats and mice) has a distinct structure of amino acids and is less likely to generate amyloid. Mice that expressed human amylin experienced amylin amyloid formation, β-cell death, and obvious hyperglycemia when insulin resistance was induced pharmacologically. In contrast to murine amylin, these pathologic alterations were reproduced in rats overexpressing human amylin. So, hyperamylinemia is a risk factor for developing type 2 diabetes at an earlier stage.
In reaction to food consumption, insulin and amylin are cosecreted by pancreatic islet β cells. Amylin response to calorie intake is nonexistent and patients with type 1 diabetes had lower amylin serum concentrations at baseline. A reduced amylin response to calorie intake may be associated with the level of β-cell damage in patients with insulin-dependent type 2 diabetes. Glucagon production in response to caloric intake is suppressed, stomach emptying is delayed, and the satiety area in the brain is stimulated to reduce caloric intake; these are key physiological activities of amylin in maintaining glucose homeostasis.
Pramlintide is prescribed to adults with type 1 or type 2 diabetes when optimum insulin therapy has not resulted in satisfactory glucose control. Pramlintide, when used in conjunction with insulin, significantly decreased A1C in type 1 and type 2 diabetic patients and had a positive impact on weight. It is usually given under the skin just before a big meal. When pramlintide is used with insulin, the risk of hypoglycemia is higher. Nausea, vomiting, anorexia, decreased appetite, headache, and other side effects are also possible. Pramlintide is most effective when prescribed after careful patient screening and education.
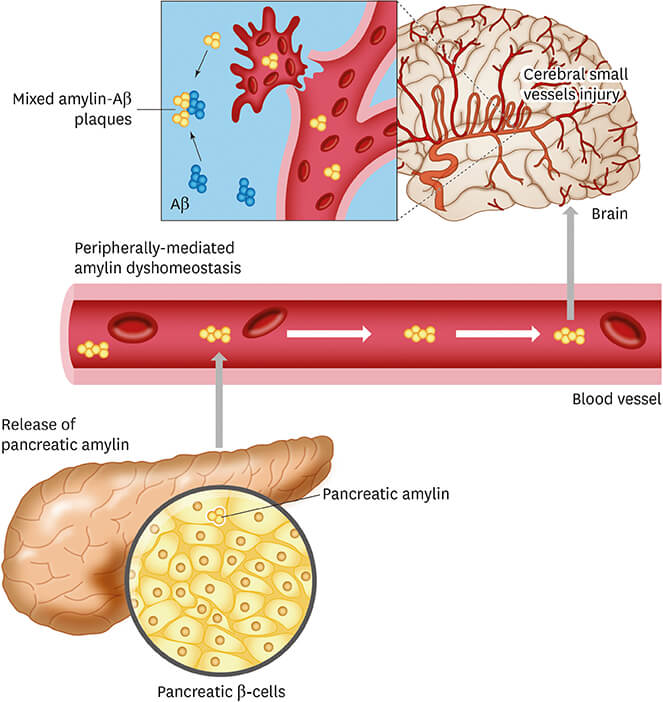
Diabetes-related amylin dyshomeostasis leads to the formation of pancreatic amyloid. (Ly H., et al., 2019)
References