At Creative Peptides, we specialize in custom Peptide-siRNA Conjugation—an advanced bioconjugation platform that unites high-purity peptide synthesis with precision siRNA modification to create multifunctional RNAi constructs with improved stability, cellular uptake, and tissue targeting. Our chemists integrate robust solid-phase synthesis, rational linker engineering, and comprehensive analytical validation to deliver reproducible, scalable peptide–siRNA conjugates tailored to your R&D, preclinical, or GMP programs. Whether you are developing targeted RNAi therapeutics, intracellular gene-silencing tools, or peptide-enabled delivery systems, we provide a reliable, cost-effective end-to-end workflow from design to production.
While siRNA is a powerful modality for sequence-specific gene silencing, clinical translation is often limited by poor cellular entry, rapid nuclease-mediated degradation, suboptimal tissue localization, and inefficient endosomal escape.
Peptide-siRNA Conjugation directly addresses these bottlenecks by:
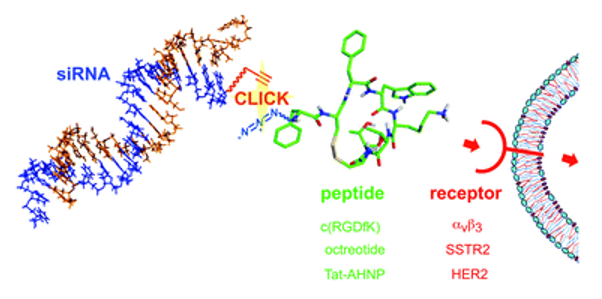 Fig. 1 Efficient siRNA-peptide conjugation for specific targeted delivery into tumor cells. (Gandioso, A., 2017)
Fig. 1 Efficient siRNA-peptide conjugation for specific targeted delivery into tumor cells. (Gandioso, A., 2017)
We provide comprehensive, end-to-end peptide–siRNA conjugation services designed to meet the requirements of enterprise research, preclinical development, and GMP manufacturing. Each service module is fully customizable and supported by scientists experienced in peptide chemistry, RNA modification, and site-specific bioconjugation—ensuring your conjugate design aligns with delivery goals, potency targets, and regulatory expectations.
Successful peptide–siRNA conjugates begin with data-driven molecular design. Our team collaborates with you to define:
We then provide a technical roadmap including risk assessment, projected yield, development timeline, and an analytical/QC plan—supporting fast, transparent decision-making from concept to delivery.
Our peptide platform uses controlled solid-phase synthesis with rigorous in-process monitoring to support conjugation-ready materials for RNA delivery.
Every peptide batch is released with documentation aligned to your project stage—research-grade through GMP-compatible deliverables.
We manufacture siRNA with tight control over sequence fidelity, strand purity, duplex formation, and modification pattern—optimized for conjugation and biological performance.
Our RNA team ensures low failure sequences, minimal side products, and conjugation-ready substrates to support reproducible manufacturing.
Using validated, site-specific chemistries, we perform controlled peptide–siRNA conjugation under optimized conditions to maximize yield while preserving duplex integrity and function.
Reactions are monitored using LC and UV profiles to ensure reproducibility, scale-readiness, and batch-to-batch consistency.
Each peptide–siRNA conjugate undergoes comprehensive purification and analytical verification to confirm chemical identity, duplex quality, and critical attributes relevant to RNAi performance.
We provide scalable solutions—from milligram discovery batches to gram-level GMP manufacturing to support IND-enabling studies and clinical supply strategies.
Our manufacturing capabilities include:
To complement chemical development, we can support biological evaluation and functional readouts through qualified partner laboratories.
Available assays:
Selecting the right peptide is critical for targeted siRNA delivery, intracellular release, and overall RNAi performance. We offer a broad portfolio of peptide options and can tailor sequences to your receptor biology, tissue strategy, and formulation constraints.
| Peptide Type | Main Function | Common Sequences / Examples | Typical Applications | Key Advantages |
|---|---|---|---|---|
| Cell-Penetrating Peptides (CPPs) | Facilitate membrane translocation and intracellular delivery of siRNA | TAT, Penetratin, R8, Transportan, Pep-1 | Intracellular siRNA delivery, screening, mechanistic studies | Enhanced uptake; can be tuned for charge and toxicity profile |
| Targeting Peptides | Direct peptide–siRNA conjugates to specific receptors/cell types | RGD, NGR, Angiopep-2, MSH analogs (example families) | Tumor targeting, CNS delivery, receptor-mediated uptake programs | Improved selectivity and tissue specificity; supports differentiated pipelines |
| Endosomal Escape Peptides | Promote endosomal disruption and cytosolic release for RNAi activity | INF7, GALA, HA2, KALA | Potency enhancement for internalizing targets; hard-to-transfect cells | Increased cytosolic delivery; improved functional knockdown |
| Nuclear Localization Peptides (NLS) | Enable nuclear transport when required for specialized RNA systems | PKKKRKV, SV40 NLS, M9 | Mechanistic studies; nuclear-targeting research constructs | Useful for niche applications requiring nuclear access |
| Mitochondrial Targeting Peptides | Direct RNA payloads toward mitochondrial compartments (research use) | MTS (e.g., COX8a-derived motifs) | Mitochondrial biology research; exploratory RNA delivery | Organelle-directed targeting capability |
| Cleavable / Responsive Peptides | Enable controlled release in response to intracellular or disease microenvironments | Enzyme-cleavable motifs, redox-sensitive elements, acid-labile designs | Tumor microenvironment-responsive delivery; safety/efficacy optimization | Tunable release and improved therapeutic window |
| Custom Peptides | Tailor-made sequences for proprietary delivery platforms | Designed per project need | Enterprise RNAi programs; platform differentiation | Fully customizable; aligned to your receptor biology and IP strategy |
Our Peptide-siRNA Conjugation platform supports a range of RNAi-relevant oligonucleotide formats used in discovery, translational research, and therapeutic development. Each format has distinct constraints that influence conjugation site selection, linker design, and peptide choice.
| Molecule Type | Full Name / Description | Typical Modifications | Applications | Conjugation Advantages |
|---|---|---|---|---|
| siRNA | Small Interfering RNA – duplex RNA (typically 21–23 nt) enabling sequence-specific gene knockdown via RNAi | 2′-O-methyl, 2′-F, phosphorothioate termini, stabilized overhangs, immunostimulation-mitigating designs | Therapeutic RNAi, target validation, functional genomics | Improved cellular uptake and targeted delivery with peptide guidance; supports endosomal escape strategies |
| DsiRNA | Dicer-substrate siRNA – longer duplex designed for Dicer processing and potent RNAi | Site-specific 2′ modifications, terminal PS, stabilized ends to optimize processing | Potency-driven knockdown programs; difficult targets | Peptide conjugation can enhance uptake while preserving processing-dependent activity |
| miRNA Mimic / Inhibitor | MicroRNA modulators – oligos that increase or inhibit miRNA function to modulate gene networks | 2′-O-methyl, LNA/MOE options (as applicable), terminal modifications for stability | Pathway modulation, oncology, inflammation research | Peptide conjugation can improve intracellular delivery and expand tissue scope |
| shRNA Mimic (Synthetic) | Hairpin-inspired RNA – synthetic oligos designed to mimic shRNA processing behavior | Stabilizing nucleotide chemistry, defined reactive handles for conjugation | Mechanistic RNAi studies; tool compound development | Enables peptide-driven uptake while supporting processing pathways |
| sgRNA / gRNA (Research) | Guide RNA – RNA guiding nuclease systems; included for research delivery exploration | Stabilizing 2′ chemistry, terminal capping, reactive handles (project-dependent) | Gene-editing delivery research and screening (non-clinical or exploratory) | Peptide conjugation can enhance cellular entry and compartment access in model systems |
| RNA Probes / Oligos | Custom RNA sequences for hybridization, labeling, and assay development | Fluorophores, spacers, affinity tags, reactive handles | Diagnostics R&D, imaging, assay validation | Enables controlled immobilization, trafficking studies, and multiplex workflows |
Site-Specific Conjugation
Controlled attachment sites and linker architectures support consistent orientation and reproducible RNAi performance.
siRNA-Focused Engineering
Designs optimized for duplex integrity, strand selection, release kinetics, and RISC compatibility.
Broad Peptide Toolkit
CPPs, targeting ligands, and endosomal escape motifs to fit diverse tissues, receptors, and delivery hypotheses.
Enhanced Delivery Efficiency
Peptide conjugation improves internalization, trafficking, and functional knockdown in relevant models.
Improved Stability
Optimized chemistries and designs extend stability in biological environments and support translational studies.
Scalable & GMP-Ready
From early discovery to GMP manufacturing with documentation and batch record support for enterprise pipelines.
Strong Analytical Package
LC-MS/HPLC-driven identity and purity verification plus optional stability and release profiling.
Fast, Enterprise-Oriented Execution
Dedicated project management, clear milestones, and reliable delivery to support program timelines.
One-Stop Delivery Solution
Integrated design, synthesis, conjugation, purification, and QC reduce vendor complexity and risk.
Our streamlined workflow ensures precision, traceability, and reproducibility across every stage—from concept to final delivery of your peptide–siRNA conjugate.
1
Project Consultation & Design Planning
2
Peptide & siRNA Synthesis
3
Conjugation & Optimization
4
Purification & Analytical Characterization
5
Scale-Up, Documentation & Delivery
Peptide-siRNA Conjugation represents a high-value delivery and differentiation strategy for RNA interference (RNAi) programs. By combining sequence-specific gene silencing with peptide-enabled targeting and intracellular transport, this technology supports a broad range of enterprise-driven applications across therapeutic development, translational research, and advanced biotechnology platforms.
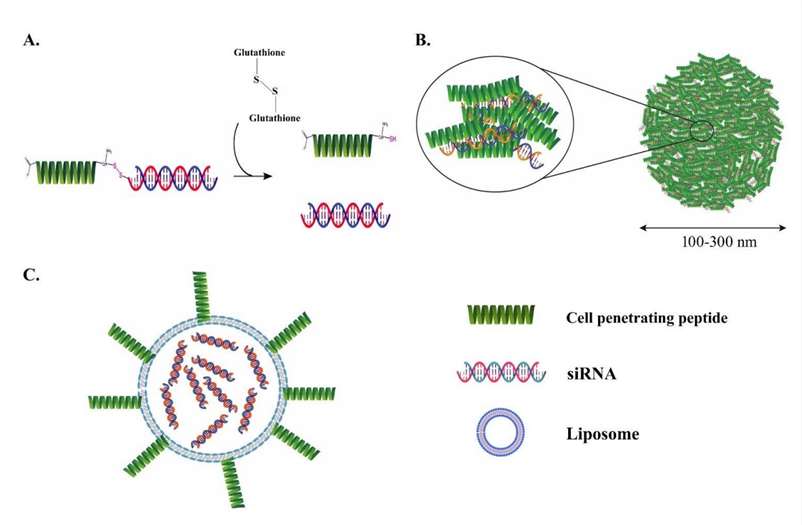 Fig. 2 CPPs for siRNA delivery. (A) Covalently conjugated siRNA with CPP. (B) siRNA complexed with the CPP and (C) CPP-decorated nanoparticle. (Ali Z., 2023)
Fig. 2 CPPs for siRNA delivery. (A) Covalently conjugated siRNA with CPP. (B) siRNA complexed with the CPP and (C) CPP-decorated nanoparticle. (Ali Z., 2023)Ready to advance your RNAi program with a scalable, analytically validated Peptide-siRNA Conjugation solution? Partner with Creative Peptides for enterprise-grade design, synthesis, conjugation, purification, and QC—supporting discovery through GMP manufacturing. Contact our team to discuss targeting strategy, linker options, analytical packages, and project timelines for your peptide–siRNA conjugate development.
The ability of peptides to target specific cell types or tissues enhances the delivery of siRNA, which plays a crucial role in gene silencing. The conjugation enhances cell uptake, improves endosomal escape, and allows targeted delivery with minimal off-target effects.
The main challenges include the efficient synthesis of stable peptide-siRNA conjugates, the selection of appropriate peptides, and the potential immune responses to dsiRNAs-peptide conjugates. Moreover, understanding the fate of peptide-siRNA conjugates inside cells and optimizing the stability in physiological conditions are also significant.
The stability is usually determined by serum stability assays, where the conjugates are incubated with serum and the amount of intact conjugate is measured over time. Another approach is the use of nuclease resistance assays, where the resistance to enzymatic degradation of the conjugate is assessed.
Two common methods are the use of covalent bonds or non-covalent bonds. Covalent methods involve chemical reactions that create a stable bond between the peptide and the siRNA, while non-covalent methods involve the use of physical forces or biological interactions.
