Creative Peptides focuses on providing comprehensive services related to peptide radioisotope conjugate (PRC) and are committed to advancing the development of precision medicine and personalized therapy.
Peptide radioisotope conjugate is a drug form that uses a radionuclide as a payload and a peptide through a linker. The highly selective ability of peptides to bind to specific disease markers, such as tumor antigens, combined with the radiation effects of radionuclides, enables targeted drug delivery. The peptide conjugate binds to the radionuclides through a stable chelating agent, ensuring a stable presence in the blood circulation until it reaches the target. The basic structure of this class of drugs consists of three key components: targeting peptides, linkers, and radionuclide loads.
Targeted peptides: as carriers, peptides can specifically recognize and bind to specific receptors or antigens on the surface of tumor cells, such as prostate specific membrane antigen (PSMA), somatostatin receptor (SSTR), carbonic anhydride IX (CAIX), etc., to achieve accurate drug delivery.
Linker: The linker is the bridge between the peptide and the rationuclide chelate (such as DOTA), and its selection is crucial because it needs to ensure not only the stable binding of the peptide to the nuclide, but also stability in the in vivo environment, avoid premature dissociation, and sometimes consider the controlled release properties under specific conditions (such as the action of enzymes).
Radionuclide loads: DOTA and other chelates can effectively bind to specific radionuclides to form stable complexes. This coupling not only enhances the stability of the nuclide in vivo, but also facilitates the precise delivery of the nuclide to the target location through the peptide carrier. Depending on the therapeutic or diagnostic needs, the nuclides used can emit different particles, such as positrons (β+, for PET imaging), gamma rays (for SPECT imaging), beta particles (for therapy), or alpha particles (high energy, highly effective in killing tumor cells). The selection of nuclides takes into account their physical half-life, penetration depth and toxicity.
Diagnostic nuclides usually emit positrons (β+), such as 18F, 68Ga, 123I, etc., or radionuclides that emit gamma photons, such as 99Tc, 67Ga. These nuclides are used for imaging by positron emission tomography (PET) or single photon emission computed tomography (SPECT). For example, 68Ga-PSMA-11 is a diagnostic reagent for PET imaging of prostate cancer that helps identify prostate cancer lesions by binding to prostate-specific membrane antigen (PSMA) with high sensitivity and specificity. The physical half-life of this class of drugs is short, generally within one to ten hours, to reduce radiation exposure to patients.
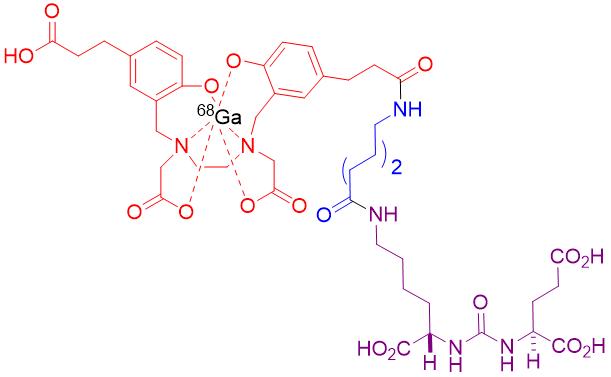 Fig. 1 The structural formula of 68Ga-PSMA-11 (purple for targeted peptide, blue for linker, red for chelating agent, black for radionuclide)
Fig. 1 The structural formula of 68Ga-PSMA-11 (purple for targeted peptide, blue for linker, red for chelating agent, black for radionuclide)
Therapeutic nuclides usually emit alpha particles (such as 223Ra, 225Ac) or beta particles (such as 90Y, 177Lu, 90Sr), which can directly damage DNA, mitochondria, and cell membranes, causing cell death. At the same time, they may also indirectly kill surrounding or distant tumor cells through bystander effect and remote effect. For example, 177Lu-PSMA-617, a treatment for metastatic castration-resistant prostate cancer, specifically targets PSMA-expressing tumor cells, releasing beta particles to destroy cancer cells. The physical half-life of therapeutic nuclides is long, generally between one day and ten days, to ensure that the drug has enough residence time in the body to exert the therapeutic effect.
Prostate cancer: 68Ga-PSMA-11 is used to diagnose metastatic and recurrent prostate cancer, and 177Lu-PSMA-617 is used to treat metastatic castration-resistant prostate cancer, both of which target PSMA.
Neuroendocrine tumors: such as 177Lu-DOTATATE is used to treat neuroendocrine tumors, targeting the somatostatin receptor (SSTR).
Other solid tumors: Debio 0228/0328, for example, uses CAIX peptide as a targeted vector for the diagnosis and treatment of clear cell renal cell carcinoma (ccRCC) and other solid tumors.
Precise targeting: As a carrier, peptides have highly specific targeting ability, and can recognize antigens or receptors specifically expressed on the surface of tumor cells, such as PSMA, SSTR, carbonic anhydride IX, etc., to achieve accurate localization of tumor cells and reduce damage to normal tissues.
Strong tumor penetration: Compared with large molecules such as antibodies, the peptide has a small molecular weight, has better tissue penetration, can reach the deep tissue of the tumor more effectively, and is especially suitable for the treatment of solid tumors.
Low immunogenicity: Due to their small molecular weight, peptide radioisotope conjugates usually do not cause a strong immune response, reducing the immune rejection and allergy problems that may occur during long-term treatment.
Versatility: Peptide radioisotope conjugates can be used both for diagnosis and treatment, achieving the integration of diagnosis and treatment, and providing patients with personalized medical solutions.
Optimized pharmacokinetic characteristics: Through rational design of linkers and selection of appropriate radionuclides, the distribution, metabolism and excretion of drugs in vivo can be regulated, the accumulation and retention time of drugs at the tumor site can be improved, and the radiation exposure of non-targeted tissues can be reduced.
We have advanced peptide synthesis technology to design and produce high quality, diverse peptide chains according to customer needs. According to customers' scientific research needs and clinical applications, we provide high purity peptides. The stability and effectiveness of peptides are improved through specific chemical and biological modifications.
Advanced proteomics, phage display and peptide synthesis techniques are used to design and optimize the structure of peptide radioisotope conjugation to achieve high targeting and excellent efficacy. We screened the preferred MSLN targeting peptides with high affinity from the constructed MSLN targeting peptide library, conjugated the preferred targeting peptides with DOTA, and further integrated radioisotope. MSLN targeting peptide radioisotope conjugate imaging in abdominal cavity has a clean background, and can accurately target tumors.
Conjugating strategy design: According to the characteristics and uses of the drug, design the optimal conjugating scheme to ensure the stability and functionality of the conjugate. New peptide radioisotope conjugates are constantly being developed to meet the therapeutic needs of patients with different tumor types.
From the initial concept to the actual implementation, we provide comprehensive support to ensure that your peptide radioisotope conjugates achieve optimal results.
Drug screening and optimization: Use our proven R&D platform to rigorously screen and optimize drug candidates.
DMPK studies: Drug metabolism and pharmacokinetics studies to evaluate drug absorption, distribution, metabolism, and excretion in the body.
Peptide radioisotope conjugates are compounds that consist of a peptide linked to a radioactive isotope. These conjugates are used for diagnostic and therapeutic purposes in nuclear medicine, particularly in targeting specific cellular receptors for cancer imaging and treatment.
Peptide radioisotope conjugates offer high specificity for targeting particular cells, such as cancer cells, thereby providing accurate diagnostic imaging or targeted radiotherapy. This specificity reduces damage to surrounding healthy tissues and enhances the effectiveness of the treatment or diagnostics.
Commonly used isotopes include Technetium-99m (Tc-99m), Iodine-123 (I-123), Lutetium-177 (Lu-177), and Yttrium-90 (Y-90). The choice of isotope depends on the intended application, whether for imaging or therapy.
These conjugates are typically administered via intravenous injection. The administration method may vary depending on the treatment protocol and the specific requirements of the diagnostic or therapeutic procedure.
The peptides used in these conjugates often include somatostatin analogs, bombesin peptides, and other receptor-specific peptides. These peptides are chosen based on their ability to bind selectively to receptors that are overexpressed in certain types of cancer cells.
A chelator is commonly used to form a stable complex between the peptide and the radioactive isotope. The chelator helps to securely attach the radioisotope to the peptide, ensuring that it remains bound during circulation and delivery to the target site.
The shelf life depends on the radioactive half-life of the isotope used and the stability of the conjugate. It can range from a few hours to several days. Proper storage conditions and handling procedures are crucial to maintaining the efficacy of the conjugate.
