| CAT# | Product Name | M.W | Molecular Formula | Inquiry |
|---|---|---|---|---|
| L-Iso-0001 | L-ABRINE (METHYL-D3) | 221.27 | C12D3H11N2O2 | |
| L-Iso-0003 | D-ALANINE (<5% L) (D7) | 96.1 | C3D7NO2 | |
| L-Iso-0004 | L-ALANINE (1-13C) | 90.09 | CH3CH(NH2)*COOH | |
| L-Iso-0005 | D-ALANINE (1-13C) | 90.09 | CH3CH(NH2)*COOH | |
| L-Iso-0006 | DL-ALANINE (1-13C) | 90.09 | CH3CH(NH2)*COOH | |
| L-Iso-0008 | L-ALANINE (1-13C; 15N) | 91.08 | *CC2H7*NO2 | |
| L-Iso-0009 | L-ALANINE (13C3, D4, 5N) | 97.09 | *CD3*CD(*NH2)*COOH | |
| L-Iso-0010 | DL-ALANINE (13C3) | 92.1 | *CH3*CH(NH2)*CO2H | |
| L-Iso-0011 | L-ALANINE (13C3) | 92.07 | *CH3*CH(NH2)*COOH | |
| L-Iso-0012 | L-ALANINE (13C3; 15N) | 93.07 | *CH3*CH(*NH2)*COOH | |
| L-Iso-0013 | L-ALANINE (15N) | 90.09 | CH3CH(*NH2)COOH | |
| L-Iso-0014 | D-ALANINE (15N) | 90.1 | C3H7*NO2 | |
| L-Iso-0015 | DL-ALANINE (15N) | 90.1 | CH3CH(*NH2)COOH | |
| L-Iso-0017 | L-ALANINE (18O2) | 93.09 | C3H7NO2 | |
| L-Iso-0018 | DL-ALANINE (2,3,3,3-D4) | 93.12 | CD3CD(NH2)COOH | |
| L-Iso-0019 | L-ALANINE (2,3,3,3-D4) | 93.12 | CD3CD(NH2)COOH | |
| L-Iso-0020 | L-ALANINE (2,3,3,3-D4; 15N) | 94.11 | C3D4H3*NO2 | |
| L-Iso-0021 | L-ALANINE (2,3-13C2) | 91.08 | *CH3*CH(NH2)COOH | |
| L-Iso-0022 | D-ALANINE (2,3-13C2) | 91.08 | *CH3*CH(NH2)COOH | |
| L-Iso-0023 | DL-ALANINE (2,3-13C2) | 91.08 | HOOC*CH(NH2)*CH3 |
Stable isotope labeled amino acids (SILAA) play an important role in the progress of protein's genomics, metabonomics, clinical diagnosis and other research applications. Creative Peptides provides a broad range of stable isotope labeled amino acids, support researchers' efforts in various professional fields, advance knowledge and expand the possibility of biopharmaceutical research.
Atoms with the same atomic number but with different atomic masses are called isotopes.
According to the different characteristics of these isotopes, they can be divided into two main types: radioactive isotopes and stable isotopes. Isotopes, which emit radioactive rays and neutrons, are called radioisotopes. While isotopes that are always stable are called stable isotopes. For example, there are three isotopes of hydrogen. Two of these isotopes 11H and 21H atoms are stable isotopes (not radioactive), while 31H tritium is radioisotope.
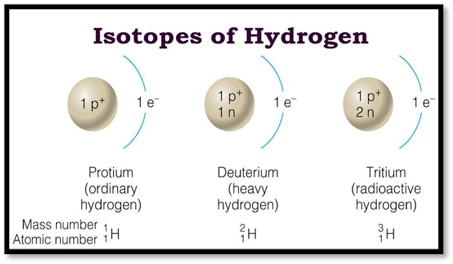 Fig. 1 Isotopes of hydrogen
Fig. 1 Isotopes of hydrogen
Likewise, the most frequent substitutions of non-radioactive amino acids are 12C by 13C, of 14N by 15N, and of 1H by 2H (deuterium). All isotopes in the products of the amino acids from Creative Peptides are stable isotopes.
SILAA are amino acids in which one or more hydrogen (H), carbon (C), oxygen (O) or nitrogen (N) atoms have been replaced by their heavy isotopes: deuterium (D), 13C, 18O or 15N. The basis of stable isotope labeling is the usage of isotopes that do not degenerate into another form or transmute through radioactive decay. Thus, making the labeling stable. For example, L- alanine, the chemical structure shown in Figure 2, amino acids in which some of the normal carbon atoms are replaced by 13C isotope, are called 13C-labeled amino acids, in which some of the nitrogen normal atoms are replaced by 15N isotope, are called 15N-labeled amino acids and so forth. There are four types of labeling elements are currently available from Creative Peptides, 18O, 15N, 13C, and 2H.
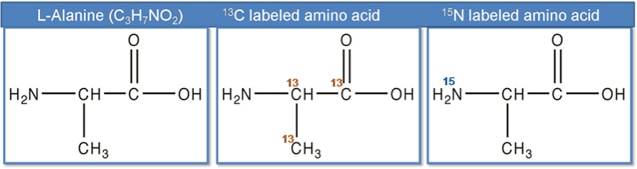 Fig. 2 Non-labeled alanine, 13C labeled alanine, 15N labeled alanine
Fig. 2 Non-labeled alanine, 13C labeled alanine, 15N labeled alanine
There are two main methods to prepare stable isotope labeled amino acids: chemical synthesis and biosynthesis.
This method involves replacing specific atomic groups such as hydrogen atoms or methyl groups in amino acid side chains. This method usually requires deuteration synthesis under acidic or alkaline conditions according to the pKa value of amino acids, through high temperature and high pressure reaction environment and multiple hydrogen-deuterium exchanges. However, this method may take a long time, consume high energy and have relatively low selectivity.
Biosynthesis is the introduction of isotopes into the physiological metabolism of animals, plants, enzymes (mainly enzymes) or microorganisms to obtain the required labeled compounds. Specifically, this method uses recombinant proteins expressed in organisms to engineer their genetic code to encode amino acids containing tagged atoms. The biosynthesis method can synthesize some compounds with complex structures and certain biological activities, but due to the types and activities of enzymes, there may be fewer deuterium-labeled amino acids that can be synthesized.
No Radioactive Hazard: Compared with radioactive isotopes, stable isotopes are not radioactive, so they will not cause radioactive hazard to human body and environment. This makes the stable isotope labeling technology safer in experimental research and practical application.
Long Half-Life: The half-life of stable isotopes is very long, even infinitely long, so the labeled samples can be stored and transported for a long time without worrying about isotope decay.
High Precision and Sensitivity: Stable isotope labeling technology has high precision and sensitivity, which can accurately track and quantify the movement and changes of molecules in organisms. This makes this technology a powerful tool to study the synthesis and metabolic pathways of protein and the interaction between drugs and biomolecules.
Wide Application: Stable isotope labeling technology can be used to study various biomolecules, including protein, nucleic acids, lipids and so on. By labeling different amino acids, nucleotides or fatty acids, we can reveal the processes of synthesis, degradation and interaction of these molecules in organisms.
Compatibility with Other Technologies: Stable isotope labeling technology can be combined with various analytical technologies, such as mass spectrometry and nuclear magnetic resonance, so as to provide more comprehensive and accurate information. This compatibility makes this technology more flexible in the study of complex biological systems.
Proteomics: SILAA have significant usage in proteomics research. They are used in quantitative proteomics to accurately compare protein expressions in different samples. They are also used in protein turnover studies to understand the dynamics of protein synthesis and degradation.
Metabolomics: In metabolomics, SILAA are used to trace the metabolic pathways, which helps understand the mechanism of metabolism and the flow of nutrients in an organism.
Disease Diagnosis: SILAA are used in mass spectrometry-based clinical diagnosis. They serve as internal standards, which provide absolute quantification of specific compounds, thereby, improving the accuracy and precision of the measurements.
Drug Development: In pharmaceutical research, these amino acids are useful for developing new therapeutic drugs and monitoring their metabolism in the body.
Environmental Studies: Stable isotope labeled amino acids are used to trace nutrient cycling and energy flow in ecosystems, helping to better understand environmental processes.
Food and Agriculture: In agriculture and food studies, they are used to study the nutrient utilization of crops, to improve food quality, and to track food adulteration.
SILAC is a technique used in quantitative proteomics, which is based on direct addition of selected stable isotope amino acids into the cell culture medium, allowing superior quantitative analysis of the cellular proteome compared to other labeling methods. The great advantages of SILAC lie in its straight-forward implementation, quantitative accuracy, and reproducibility over chemical labeling or label-free quantification strategies, favoring its adoption for proteomic research.
In this technique, proteins are labelled with a particular isotope, either heavy or light, by growing cells in a media that contains a specific form of a certain amino acid. Over time, this labeled amino acid gets incorporated into the proteins during protein synthesis. During protein analysis, proteins from the SILAC labeled cells are mixed with proteins from normally grown cells. The proteins are digested into peptides and analyzed using MS. The SILAC labeled peptides can then be distinguished from the normal peptides by their different mass/charge ratios.
Ideally, SILAC amino acids should be essential for the survival of cultured cells, so as to ensure that the only source of specific amino acids is the culture medium. Leucine, lysine and methionine are essential amino acids and have been used in SILAC.
In the early research of SILAC, deuterium labeled leucine was selected as the labeled amino acid. However, the chromatographic shift of deuterium-labeled peptides during reverse phase chromatography affects the accuracy of quantification. Later, amino acids labeled at 13C or 15N were used, because these SILAC peptide pairs would co-elute in LC-MS/MS analysis.
Although arginine (R) is not an essential amino acid, it has been proved to be essential for many cultured cell lines and has been successfully used for SILAC labeling. Because the protein must be cut into peptide segments by trypsin before the subsequent protein MS analysis, Trypsin specifically cuts from lysine (K) and arginine (R) residues in protein, and finally more than 95% of the peptide segments end in K and R, which makes the quantitative information of mass spectrometry more abundant and the quantitative results more accurate. Therefore, more and more researchers use the combination of 13C and 15N labeled arginine and lysine as labeled amino acids.
Tyrosine is another non-essential amino acid used in SILAC. The substrate of tyrosine kinase was identified by re-labeling tyrosine to study the dynamic changes of protein tyrosine phosphorylation.
Most proteins in cells do not function alone, but perform particular cellular activities through interacting with other specific proteins, or through forming protein complexes. Protein-protein interactions (PPIs) play key regulatory roles in various physiological processes, such as signal transduction, cell growth and proliferation, DNA replication, transcription and translation, and metabolism. Therefore, identification of PPIs at a global level will provide important insights into the regulation of cellular processes. Quantitative proteomics based on SILAC can be used to identify specific interacting proteins in exogenous PPIs, endogenous PPls, or induced PPls.
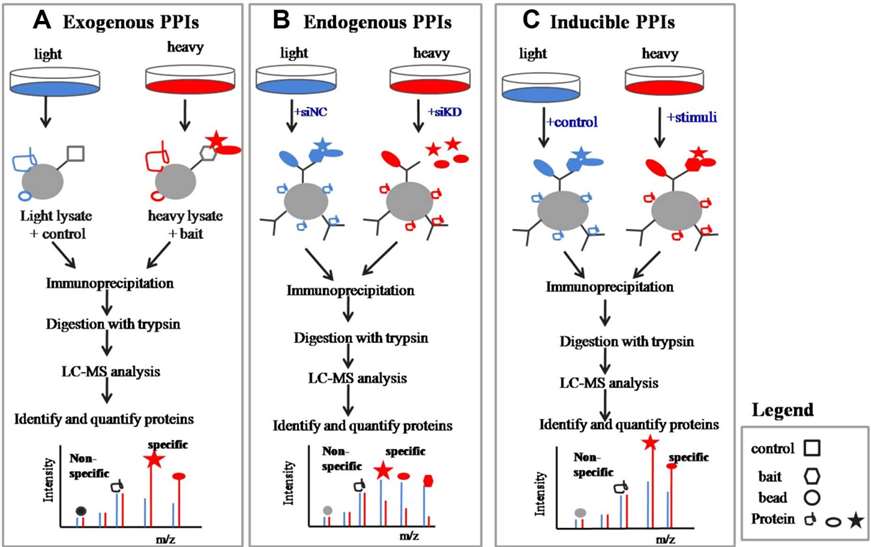 Fig. 3 Quantitative interaction proteomics with SILAC. (Chen, X., 2015)
Fig. 3 Quantitative interaction proteomics with SILAC. (Chen, X., 2015)
Creative Peptides offers a full line of stable isotope labeled amino acids for full supporting your research in this area. We can provide more than 690 stable isotope labeled amino acids include amino acid mixtures as well as all twenty amino acids with a variety of labeling patterns for all of your research needs. And we also provide stable isotope labeled peptides services, from research purity to cGMP grade. To request pricing please contact us or fill out an inquiry form below. Can't find the item you are looking for? Please inquire.
References