Since the last century, peptides have been widely accepted as drugs. Nearly 100 kinds of peptides have been sold on the market, and a large number of them are under development. In this context, peptide synthesis as a strict field of global pharmaceuticals has been greatly developed. Classical solution peptide synthesis (CSPS), solid phase peptide synthesis (SPPS) and liquid phase peptide synthesis (LPPS) have made great contributions to this field. LPPS combines the advantages of CSPS and SPPS, where the peptide elongation is carried out in solution, and the growing peptide chain is supported on the soluble tag, which gives the characteristic. The LPPS program allows large-scale production of peptides and reduces the use of excessive reagents and solvents, thus meeting the principles of green chemistry.
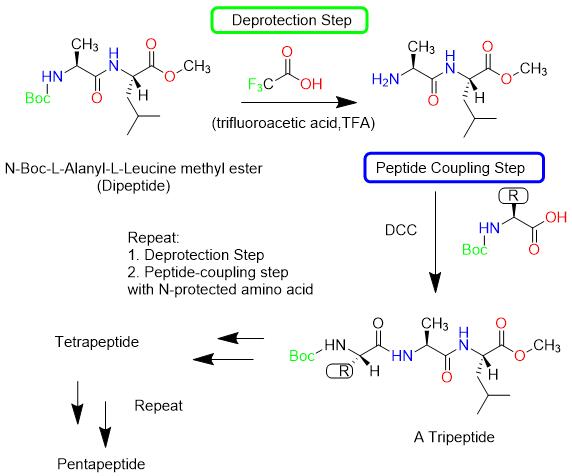
Liquid phase peptides synthesis is the classical method used by scientists for the first time to discover how to produce peptides in vitro, and it is still widely used in large-scale synthesis. Liquid phase synthesis is to configure amino acids or peptides into a homogeneous solution, take N-protected amino acids as the starting amino acids, protect the unreacted groups, activate the reactive groups, carry out the synthesis reaction, and link the amino acid residues step by step. The peptides were synthesized and purified to remove unreacted substances. This method can also be carried out by piecewise synthesis, purification in each step, and finally the synthesis of the target peptide drug. One of the advantages of liquid phase synthesis is that since the product is purified after each step, the side reaction can be easily detected.
Natural chemical ligation is the most simple and practical piecewise connection method. In 1994, Kent et al. added C-terminal thioester peptide and N-terminal Cys (cysteine) to phosphate buffer at pH 7.6 to obtain the peptide with Cys as the connecting site. Using natural chemical ligation technology, Shin et al synthesized glycoproteins with antibacterial activity; Tam et al. produced cyclic proteins containing multiple Cys residues; Duhee et al successfully synthesized proteins with 46 amino acid residues by two-stage and tertiary condensation; Regula et al synthesized proNPY with 69 oxygen-acid residues by this method. Gapbor et al synthesized the first semisynthetic serine protease SGT by this method.
Chemo and regioselective ligation is a Cys site ligation technique in which 2-mercapto benzyl acts as an auxiliary group to produce nucleophilic reactions with thioesters. The introduction of electronic groups can improve the nucleophilicity and electron concentration on the aromatic ring, thus improve the rate-limiting step, the rate of sulfur transesterification reaction and the sensitivity of auxiliary groups to acid, and greatly accelerate the condensation rate between chain segments, which is beneficial to the removal of auxiliary groups.
The peptide fragments concentrated by chemical regioselective ligation are non-side chain protected fragments. In synthesis, strong acids such as HF must be used to remove side chain protective groups and resins. However, the peptide of this product is unstable to acid and is easily affected by strong acid, so it is difficult to synthesize N-terminal peptide.
Staudinger et al. developed the Staudinger binding method based on biochemical azide reaction, which is the basic method of peptide condensation. Stoudenger connection is the reaction of C-terminal phosphine thioester and N-terminal azide to form phosphine hydroimine, which is an intermediate with nucleophilic nitrogen. The hydroimine phosphide was rearranged in the molecule to obtain amide phosphine salt, which was hydrolyzed to obtain target peptide and phosphine oxide.
Maarseveen et al. synthesized cyclic n-lactylaniline with 7-9 amino acid residues. Baddeley and others also proved that this method is an effective method of fragment condensation and broadens the field of fragment condensation.
Compared with solid phase synthesis, liquid phase synthesis has the advantages of more protective groups, low cost, easy amplification and so on. The disadvantage is that the synthetic range is small. However, in recent years, scientists have made some breakthroughs in the field of liquid phase peptides synthesis, which makes liquid phase peptide synthesis still occupy a dominant position in some fields.
References