2024-07-09
Introduction
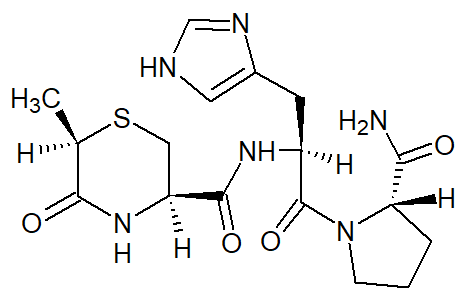
Figure 1. The structural formula of montirelin
Montirelin, an analog of thyrotrophin-releasing hormone (TRH) is more potent and acts longer than TRH, producing beneficial effects in animal models of concussion-induced unconsciousness, cerebral ischemia, memory disruption, spontaneous convulsions in rats, narcolepsy, and spinal trauma. Synthesized by the neurons in the paraventricular nucleus of the hypothalamus, montirelin stimulates the release of thyrotropin and prolactin. It is also a known compound that stimulate central nervous system, which has been suggested for possible use as an anti-depressant or in the treatment of loss of consciousness caused by head concussion. Given its efficacy, the potential indications are broadened to include seizures, nerve trauma, cognitive dysfunction, and sleep apnea.
Pharmacologic action
According to thyrotropin-releasing hormone (TRH) receptor binding after intravenous (IV) injections of montirelin, montirelin inhibits specific [3H]-Me-TRH binding and reduces the [3H]-Me-TRH binding sites. On the other hand, the IV injection of agent has little significant effect on the apparent dissociation constant for [3H]-Me-TRH. After the IV injection of montirelin, there is a counter-clockwise hysteresis between the plasma concentration and receptor occupancy of the agent. Montirelin exerts a fairly potent effect following sustained occupation of brain TRH receptors under in vivo condition.
Function
Montirelin is effective for the treatment of seizures, anesthesia recovery and loss of consciousness, by acting at a 10-fold lower dose for a longer time than natural TRH. The overall clinical status score of 73% of the patients with consciousness disorders over 14 days was improved in studies.
Pharmacokinetics and metabolism
The metabolism of montirelin hydrate (NS-3) is studied after intravenous administration of 14C-labeled or unlabeled NS-3. Four radioactive metabolites (M-1 to M-4) are found in the urine after administration of 14C-NS-3. M-3 (major metabolite) and M-2 are purified from the urine after administration of unlabeled NS-3. Consequently, M-3 is identified as (-)-N-[[(3R,6R)-6-methyl-5-oxo-3-thiomorpholinyl] carbonyl]-L-histidyl-L-proline (CNK-6004) formed by deamidation at a prolinamide moiety of NS-3, and M-2 as (+)-N-[[(3R,6R)-6-methyl-5-oxo-3-thiomorpholinyl] carbonyl]-L-histidine (CNK-6001) formed by deprolination of CNK-6004.
References:
1. Urayama, A., Yamada, S., Hirano, K., Deguchi, Y., & Kimura, R., Brain receptor binding characteristics and pharmacokinetic-pharmacodynamic analysis of thyrotropin-releasing hormone analogues. Life sciences, 2001. 70(6), 647-657.
2. Jantas, D., Tyreoliberin (Trh)-the regulatory neuropeptide of CNS homeostasis. Adv. Cell Biol., 2010. 2.
3. Haruhiko, K., Ichiro, N., Sachio, N., Clinical effectiveness of montirelin hydrate (NS-3) in patients with disturbance of consciousness. Effectiveness, safety and effect on electroencephalogram. J. Clin. Ther. Med. 1996. 12, 1129–1144.
4. Fröhlich, E., Wahl, R., The forgotten effects of thyrotropin-releasing hormone: metabolic functions and medical applications. Frontiers in Neuroendocrinology. https://doi.org/10.1016/j.yfrne.2018.06.006.