Three of the most important necessary amino acids are leucine, isoleucine, and valine, which are the components that make up branched-chain amino acids (BCAAs). Na+-dependent transporters of the apical membrane of intestinal epithelial cells are responsible for the uptake of digested BCAAs in the intestinal lumen after the ingestion of a protein-rich meal with a high protein content. The L-type Na+-dependent cotransporter LAT1 and its heterodimeric partner 4F2hc are primarily responsible for transporting them across the cell membranes of tissues. After that, they are released into the circulation by facilitated transport. Glucose homeostasis, muscle protein synthesis, and weight management are all areas that may benefit from BCAA supplementation or a diet high in BCAAs. One very significant food signal is leucine, a BCAA whose blood levels rise after a protein-rich diet. Regardless of these impacts on metabolic health, research has shown that elevated fasting concentrations of circulating BCAAs, along with blood sugar, insulin, and specific inflammatory markers, are linked to an increased risk of type 2 diabetes mellitus (T2DM) and insulin resistance in both humans and certain animal models.
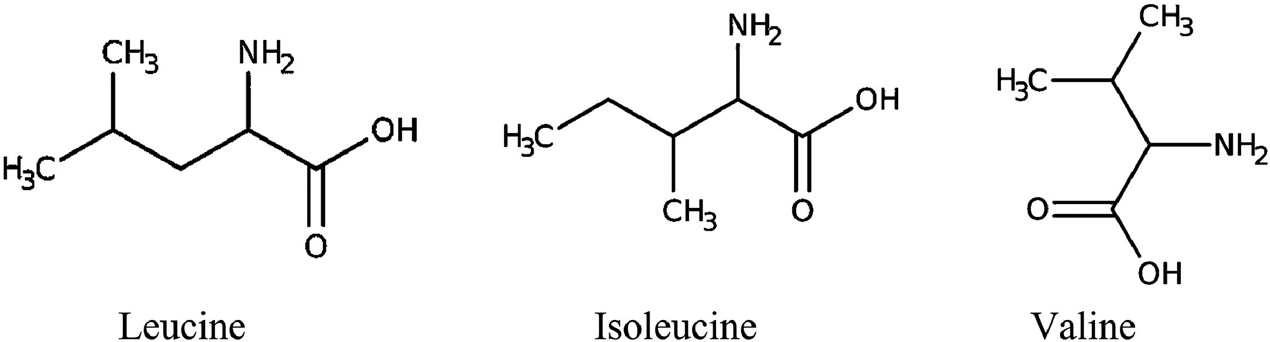 The structures of BCAAs. (Kim W K., et al., 2022)
The structures of BCAAs. (Kim W K., et al., 2022)
Metazoans are unable to produce BCAAs. In spite of this, they make up a large portion of animals, making up over 35% of the amino acids that mammals need. All three BCAAs include functional R groups that are hydrophobic, tiny, and branched—the reason for their name. As a result, these groups are essential for the majority of proteins. Among proteins found in a wide variety of organisms, BCAAs make up about 63% of the hydrophobic amino acids and 18% of the total amino acids. Due to their interdependent synthesis and oxidation processes, BCAAs typically have a molar relative abundance of around 1.6:2.2:1.0 Val:Leu:Ile. For this reason, the three BCAAs are almost always taken in tandem.
Leucine is classified as a non-polar aliphatic amino acid because it possesses an α-amino group, an α-carboxylic acid group, and a side chain isobutyl group. The protonated −NH3+ form of the α-amino group is present under biological conditions, while the deprotonated −COO− form is present. Isoleucine is present in protein in just one of its four stereoisomers, and it contains asymmetrical centers at the α- and β-carbons. Within water-soluble globular proteins, the large side chains often bind together. The polymer's three-dimensional structure is thereby stabilized by the hydrophobic amino acid residues. Valine comprises an α-amino group (existing in the protonated −NH3+ form under biological circumstances), an α-carboxylic acid group (present in the deprotonated −COO− form under biological conditions), and an isopropyl side chain, categorizing it as a non-polar aliphatic amino acid. Valine has a substantial, very hydrophobic side chain. It is a vital amino acid for humans (typical people need 0.7 g/day) and may be digested in muscle tissue.
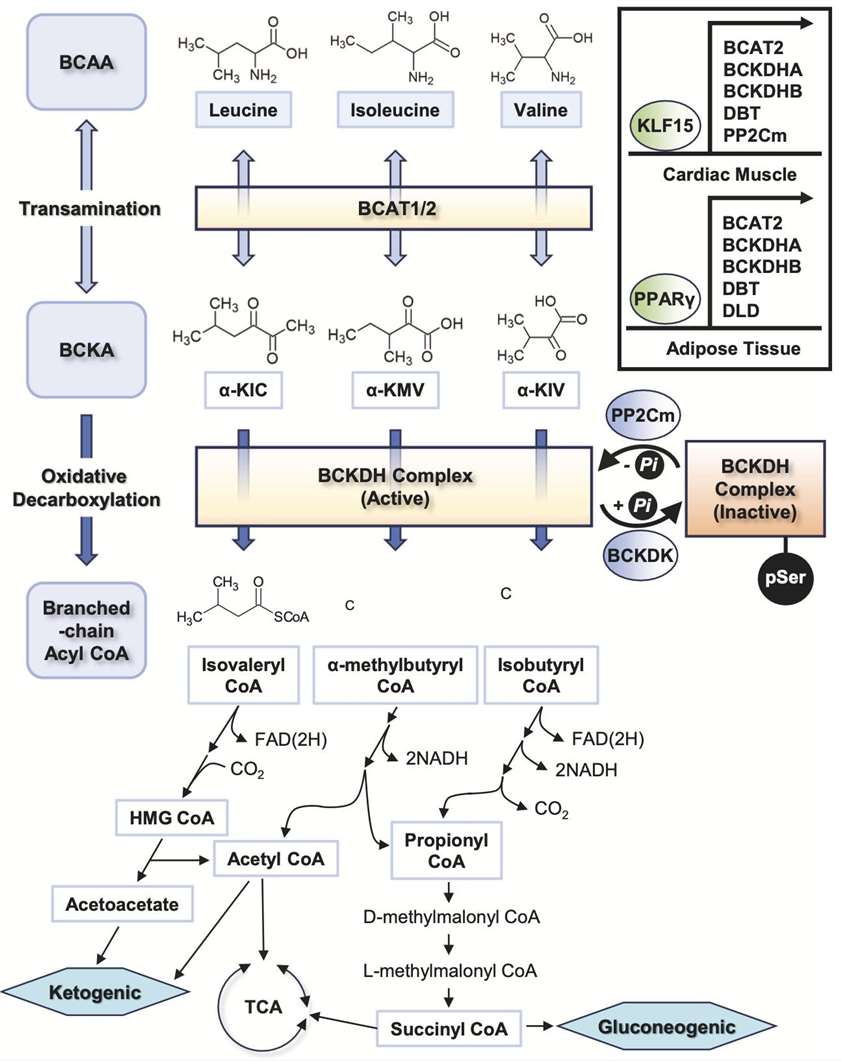 The main steps of BCAAs catabolism. (Choi B H., et al., 2024)
The main steps of BCAAs catabolism. (Choi B H., et al., 2024)
At physiological pH (~7.4), BCAAs exist as zwitterions, with the carboxyl group deprotonated (-COO-) and the amino group protonated (-NH3+). Leucine's α-carboxyl group pKa=2.3, while α-amino group pKa=9.6. Isoleucine's α-carboxyl group pKa=2.3, α-amino group pKa=9.7. Valine's α-carboxyl group pKa=2.3, α-amino group pKa=9.6.
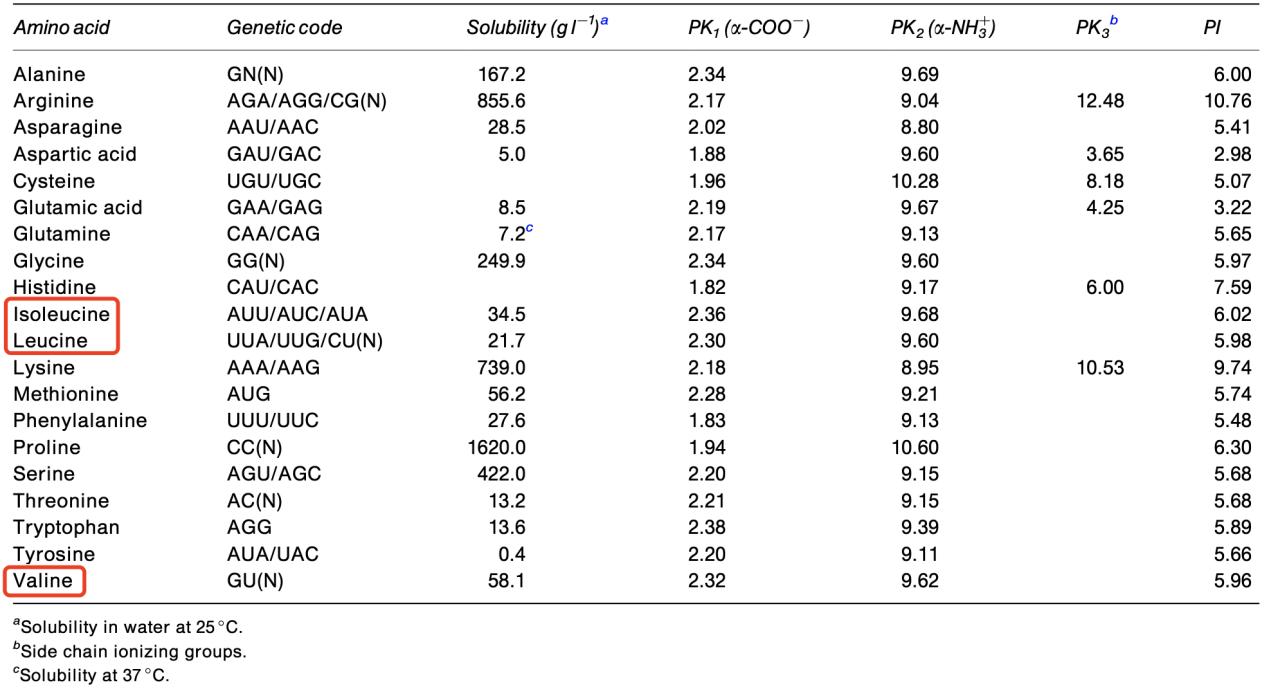 Genetic code and some physicochemical properties of the common amino acids. (Amaya-Farfan J., et al., 2003)
Genetic code and some physicochemical properties of the common amino acids. (Amaya-Farfan J., et al., 2003)
Essential amino acids (EAAs) include nine amino acids that the body is incapable of synthesizing and must be acquired from dietary sources, including histidine, lysine, methionine, and tryptophan, among others. BCAAs are amino acids characterized by an aliphatic side chain including a central carbon atom bonded to three or more carbon atoms. Among the proteinogenic amino acids, three are branched-chain amino acids: leucine, isoleucine, and valine. The three proteinogenic BCAAs are classified as EAAs for humans, comprising 35% of the essential amino acids in muscle proteins and 40% of the preformed amino acids necessary for mammals.
EAAs are crucial for protein synthesis, enzyme function, and hormone production, while CAAs especially enhance muscle metabolism, energy generation, and recuperation. In contrast to other essential amino acids, branched-chain amino acids are digested directly in muscle tissue instead of the liver, rendering them favored in sports nutrition. While all BCAAs are EAAs, not all EAAs are BCAAs.
All forms of life follow a similar metabolic pathway for BCAAs. In mammals, branched-chain amino transferases (BCATs) transaminate BCAAs to create branched α-ketoic acids (BCKAs). Just to clarify, leucine becomes α-ketoisocaproic acid (KIC), isoleucine becomes α-ketomethylvaleric acid (KMV), and valine becomes α-ketoisovaleric acid (KIV). The glutamate-producing α-ketoglutaric acid (αKG) is the nitrogen receptor that is most often found. Due to its rapidity and relatively constant free energy, the reaction is likely to remain stable in the vast majority of instances. The BCAT gene family consists of two copies: one, BCAT1 (or BCATc), encodes proteins localized to the cytoplasm and expressed mostly in the brain, while the other, BCAT2 (or BCATm), produces proteins specific to the mitochondria and expressed ubiquitously throughout many different tissues. The complex of branched-chain amino acid dehydrogenase (BCAH) is another enzyme that is critical for BCAA metabolism. The presence of BCKDH on the inner membrane of mitochondria makes it an important role in regulating the breakdown of BCAAs and a rate-limiting enzyme in BCAA catabolism. The irreversible oxidation and decarboxylation of BCKAs occurs under the catalysis of the BCKDH complex. This process releases CO2 and covalently adds coenzyme A (CoA) to the oxidized BCKA products. Decarboxylation of BCKDH yields the branched acyl CoA derivatives that match. A portion of the carbon atoms in BCAAs are eventually wasted as CO2, while the remaining portion is recycled in the tricarboxylic acid cycle (TCA cycle). Another important regulator in BCAA metabolism is BCKDH kinase (BCKDK) or phosphatase (PPM1K), which either promotes or inhibits BCKDH activity.
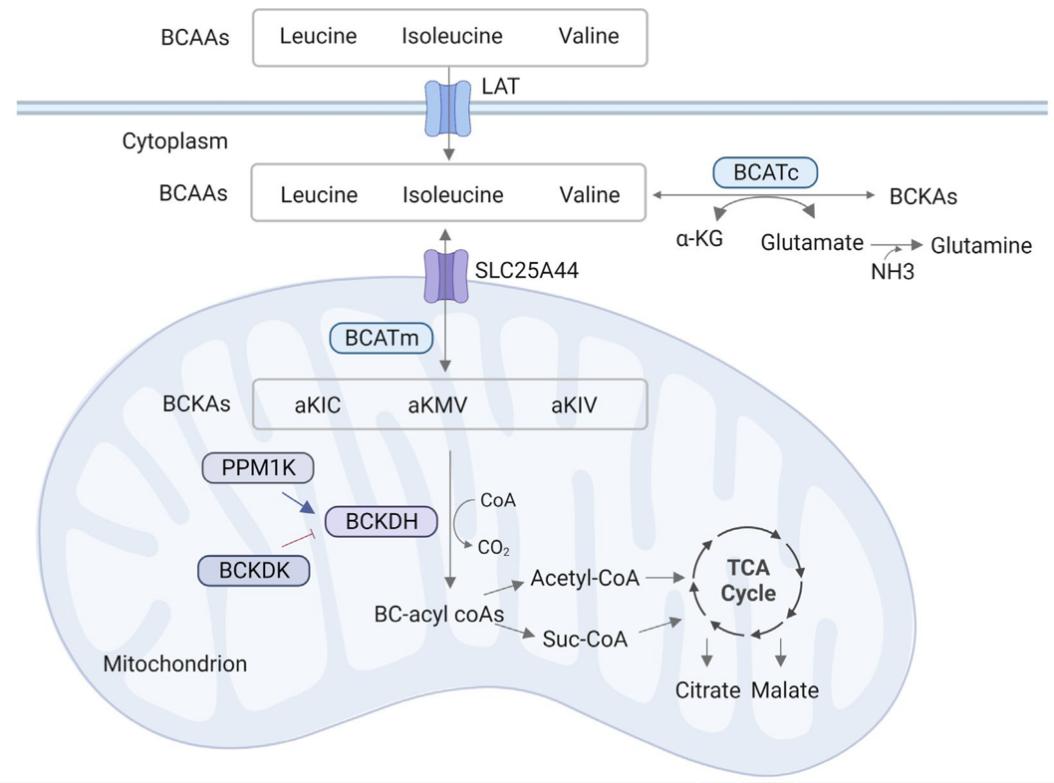 The metabolism of BCAAs. (Zhang X., et al., 2023)
The metabolism of BCAAs. (Zhang X., et al., 2023)
The BCAAs acted as signaling molecules in nutrition metabolism in addition to their roles as nutritionally EAAs for protein synthesis. In addition to regulating BCAA levels, adipose tissue stores surplus calories and facilitates lipolysis, two processes that contribute significantly to glucose and lipid homeostasis. Raised BCAAs catabolism and inhibited fatty acid oxidation in skeletal muscle and adipose tissue, respectively, are consequences of obesity and excessive food consumption. Prior research has shown that adipose tissue may be able to control BCAA levels in the bloodstream in living organisms by coordinating the regulation of BCAA enzymes within adipose tissue. Another research found that young, developing mice whose BCAA intake was reduced had better metabolic health overall. This included improved glucose tolerance, somewhat slower fat mass increase, and quicker reversal of diet-induced obesity. An important step in this process is the temporary activation of the hormone fibroblast growth factor 21, which regulates energy balance. Calorie restriction and increased exercise did not govern weight normalization; instead, it was regulated by increased energy expenditure. The role of glutamine as a Leu metabolite is vital in many physiological functions, including energy balance, cell proliferation, and apoptosis. In HepG2 cells, it has the potential to activate the fatty acid β-oxidation pathway. Glutamate deprivation may control lipid metabolism by activating the fatty acid β-oxidation pathway, as shown by this research.
Through the phosphatidylinositol 3-kinase (PI3K)-AKT (often called protein kinase B, PKB) pathway, BCAAs can control glucose and lipid metabolism. In cellular signaling pathways, the PI3K acts as a nuclear factor that is involved in several biological activities and is essential for cellular survival, development, and immunity. According to the signal transduction study, Ile is not reliant on mTOR but rather facilitates glucose absorption via PI3K. Ile inhibits the development of visceral adiposity and hyperinsulinemia, promotes glucose absorption in skeletal muscle, and stops plasma glucose concentrations from rising. Oral glucose tolerance testing in healthy rats shows that Ile, when compared to Leu and Val, considerably lowers plasma glucose levels. When white adipose tissue does not get enough BCAAs, its lipid metabolism alters drastically. White adipose tissue (WAT) fat mobilization is enhanced and hepatic lipogenesis is suppressed in leu deprivation, and the same is true for Val or Ile deficit in lowering fat accumulation.
A signal sensor for regulating energy balance, AMP-activated protein kinase (AMPK) may have its activity inhibited by dietary supplementation with leu. In female broiler chickens, the AMPK-mTOR-FoxO1 pathway is probably responsible for the regulation that leads to reduced BCAA levels, which in turn reduces fatty acid synthesis and enhances fatty acid β-oxidation. The oxido-glycolytic skeletal muscle's uncoupling protein 3 (UCP3) mRNA level might be enhanced by supplementing a protein-restricted diet with the ideal BCAAs ratio (Leu:Ile:Val = 1:0.75:0.75-1:0.25:0.25). The translocation of glucose transporter 4 (GLUT4) is one mechanism by which UCP3 affects glucose uptake, and its abundance is inversely correlated with skeletal muscle glucose metabolism. Furthermore, the neuronal cell's mitochondrial respiration and energy metabolism may be inhibited by branched-chain α-keto acids (BCKA), a byproduct of BCAAs. On the other hand, BCKA can shield mitochondria and energy generation from oxidative damage. There is a dearth of evidence supporting the idea that β-hydroxy-β-methylbutyrate (HMB) regulates lipid metabolism. Glycemic control and insulin production are improved in diabetic rats when liraglutide is supplemented with glutamine, a metabolite of BCAAs. This improvement is accompanied by an increase in the expression of sodium-dependent neutral amino acid transporter-2, a pancreatic protein that regulates insulin and glucagon secretions. These genes, which are related to the function of adipose tissue, include AMPKα, mTOR, silent information regulator transcript 1 (SIRT1), and peroxisome proliferator-activated receptor-g coactivator-1α (PGC-1α), could be regulated by changing the ratios of BCAAs. The AMPK-mTOR pathway, the Sirt1-AMPK-PGC-1α axis, and mitochondrial biogenesis may all regulate these effects. An important role for the transcription factor Krüppel-like factor 15 (KLF15) in controlling glycemic, lipid, and AAs metabolism in many cells, particularly BCAAs metabolism, is well-established. According to a new research, KLF15 expression was downregulated by high BCAA concentrations and upregulated by BCAA deprivation.
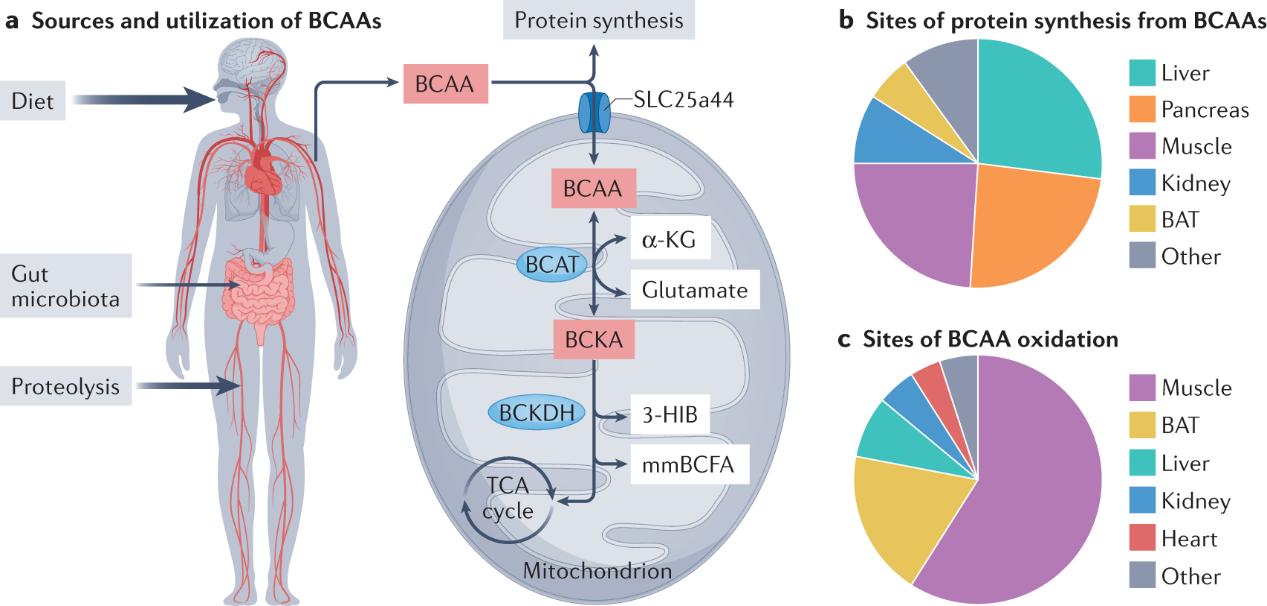 Major sources and sites of BCAA supply and utilization. (McGarrah R W., et al., 2023)
Major sources and sites of BCAA supply and utilization. (McGarrah R W., et al., 2023)
Leu is the most essential protein in skeletal muscle, and BCAAs increase protein synthesis in vitro by upregulating the beginning of mRNA translation, which is regulated by the mTOR pathway and includes TSC2, Rheb, and raptor. Protein synthesis in skeletal muscles can be stimulated in vivo by Leu, however insulin could be required for this process. Adipose tissue is one among the many tissues that Leu improves protein synthesis in; skeletal muscle is only one of them. When it comes to regulating protein synthesis in skeletal muscles, either Ile or Val on their own is ineffective. Leu does not increase global rates of protein synthesis in the liver when taken orally, but it does activate mTOR signaling and induce phosphorylation of 4E-BP1 and S6K1. Supplementing food-deprived mice with low doses of Leu improved their fat-loss results and significantly increased muscular protein synthesis. A mixed macronutrient beverage with a low-protein 6.25 g dosage of Leu may promote myofibrillar muscle protein synthesis in men just as well as a high-protein 25 g dose. Leu, which is created by lysosomal proteolysis, has the ability to activate mTORC1, which in turn may boost muscle protein synthesis when taken as a supplement. By promoting the start of translation, leu enhanced protein synthesis in muscle. On top of that, a new study found that those on low-protein diets who use BCAA supplements had higher net AAs fluxes throughout skeletal muscle in vivo. The reduction in 3-methylhistidinein content in the biceps femoris muscle was linked to the rise in concentrations of BCAAs, free fatty acids, and venous metabolites, such as BCKA and intramuscular plasma, and the higher net AAs fluxes.
Extensive research has shown that Leu plays an essential role in protein biosynthesis, protein degradation inhibition, protein activity enhancement, and the availability of certain eukaryotic initiation factors. The phosphorylation of S6K1, 4E-binding protein 1 (4E-BP1), and Eukaryotic initiation factor 4E (eIF4E) assembly are all steps in the mTOR signaling pathway that BCAAs, and Leu in particular, activate, modulating protein activity in mRNA translation. Researchers found that after resistance exercise, people whose diets consisted solely of BCAAs (i.e., without EAAs, intact protein, or other macronutrients) produced 22% more muscle myofibrillar protein synthesis than those whose diets included a placebo. This was due to an increase in the phosphorylation status of S6K1 and PRAS40, which was brought about by activating cell mTOR signaling pathways. Increased ASCT2 protein expression and enhanced mRNA expression of the Na+-neutral AA exchanger 2 (ASCT2) may be results of 7.5 mM Leu's presence. In IPE-J2 cells, leu, but not isoleucine or valine, triggered phosphorylation of 4E-BP1 and eIF4E via the activation of PI3K-AKT-mTOR and ERK signaling pathways. According to a recent prospective research, the activation of the AMPK-SIRT1-PGC-1α axis is likely responsible for the induction of muscle protein metabolism in developing pigs fed low-protein diets supplemented with the appropriate BCAAs ratio (1:0.75:0.75-1:0.25:0.25).
Researchers have recently zeroed in on how BCAAs influence immune system activities. It has been shown that immune cells have the ability to oxidize BCAAs, express the enzymes branched-chain alpha keto acid dehydrogenase and decarboxylase, and even integrate BCAAs into proteins. Lymphocytes have the highest levels of ile, followed by eosinophils and neutrophils. Leu may enhance its own breakdown by boosting the activity of lymphocyte branched-chain keto acid dehydrogenase. BCAAs play a crucial role in immune cell function as nitrogen donors and carbon skeleton suppliers for other amino acid synthesis, such as glutamine. A lack of BCAAs in the diet makes the body more vulnerable to infections because it reduces the number of white blood cells and lymphocytes, which weakens the innate immune system. In cirrhotic individuals, restoring host defense systems, such as neutrophil phagocytic function and natural killer cell activity, may be possible with BCAA supplementation. In addition to boosting host immunity, BCAAs may bolster mucosal surface protection by stimulating SIgA production and preventing pathogen introgression into lamina propria.
A number of immune system processes are controlled by BCAAs. These processes include the production of intestinal immunoglobulins, innate and adaptive immunological responses, pro-inflammatory cytokines, dendritic cell activity, and fuel supplies for immune cells (CD4+, CD4+/CD8+). The injection of Ile via the airway resulted in a notable rise in β-defensins 3 and 4, together with reduced bacillary loads and tissue damage. This suggested that Ile may be used as a new kind of immunotherapy for infectious diseases. Also, after an immunosuppressive long-distance intense workout, BCAA supplementation restores the ability of peripheral blood mononuclear cells to proliferate in response to mitogens. Plasma glutamine concentration impacts lymphocytes, natural killer cells, and lymphokine-activated killer cells. Val enhanced the allostimulatory ability and IL-12 production of dendritic cells in a dose-dependent manner, whereas Leu removal reduced p70S6K expression. Dendritic cells from both healthy volunteers and hepatitis C virus (HCV) cirrhotic patients exhibited this effect. Increasing levels of dietary ile improved the immune response in fish, while decreasing levels of occludin, claudin-3, claudin-7, tumor necrosis factor-α (TNF-α), interleukin 10 (IL-10), Kelch-like-ECH-associated protein 1 (KEAP1), and extracellular signal-regulated kinase 1 (ERK1) were observed in the fish intestine. Ile also increased the relative mRNA expression of TOR and transforming growth factor β2 (TGF-β2) and TOR in the head kidney. Studies on septic rats using enteral feedings supplemented with parenteral glutamine showed that the amino acid reduced inflammatory cytokine production, reduced lymphatic organ death, and enhanced immunological function. Glutamate, when included in sea cucumber diets at appropriate amounts (0.4-0.8%), improves intestinal function by increasing the height and density of villus in the gut, as well as specific digestive enzymes. In juvenile turbot infected with Edwardsiella tarda, glutamine may also greatly enhance nonspecific immune responses.
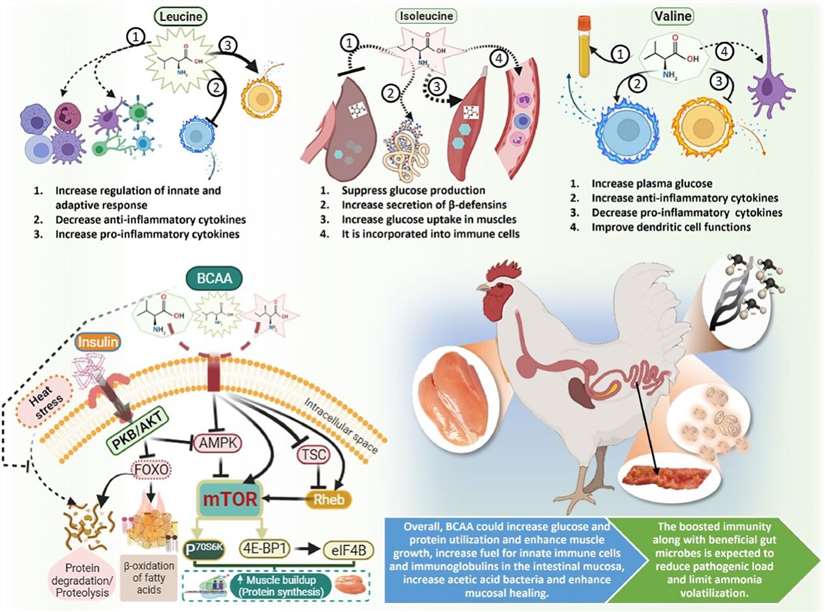 Functions of individual and mixed branched-chain amino acids. (Kim W K., et al., 2022)
Functions of individual and mixed branched-chain amino acids. (Kim W K., et al., 2022)
A reliable and repeatable method for manipulating amino acid concentrations is required for studies examining the acute regulation of protein metabolism by these compounds. This can be accomplished by indexing the infusion rate of an amino acid solution according to real-time plasma concentrations. Liquid chromatography provides precise measurements of branched-chain amino acid contents; however, analyses using these methods typically take several hours to finish. A chromatographic method that measures leucine concentrations in just five to seven minutes was published by Brown's group.
The enzyme activity units used to make the enzyme solution are the ones provided by the manufacturer. That is, in a solution with 18 mM L-leucine, 1.1 mM NAD+, and 0.18 M glycine-KCl-KOH buffer, pH 10.5, at 37°, 1 unit of enzyme activity results in the synthesis of 1 μmol of NADH per minute. We separated the lyophilized leucine dehydrogenase into manageable aliquots and stored them at -20°. Upon receiving the enzyme from the manufacturer, we diluted it in 25 mM sodium phosphate buffer, pH 7.2, to a concentration of 1 mg of protein per milliliter. This enzyme is ideal for long-term investigations since, after additional dilution for the assay, its activity remains stable for at least 24 hours when kept on ice. The glycine-KCl buffer is kept at room temperature, whereas the NAD is likewise kept on ice.
All spectrophotometric measurements are conducted at ambient temperature using a UV spectrophotometer fitted with an adjustable microcuvette cell holder and 1-ml quartz microcuvettes. The adjustable cell holder permits a reduction of the reaction volume to 300 μl, hence enhancing the assay's sensitivity. The reaction mixture comprises 270 μl of assay buffer, 4 mM NAD (10 μl), and 10 μl of plasma sample. The optical density without the enzyme is around 0.22. The absorbance prior to enzyme addition must remain steady (± 0.002); this is verified for each sample by measuring the absorbance for 30 seconds before the enzyme is added. The background noise of 0.002 optical density (OD) units restricts measurements to around 0.02 OD units, which corresponds to 100 μM of branched-chain amino acids in a sample. The reaction commences with 2 units of leucine dehydrogenase (10 μl), and the optical density is assessed after 1 minute. The temporal progression of the reaction. The introduction of the enzyme alone does not alter absorption in the absence of branched-chain amino acids. The amino acid content in the sample is determined using the extinction coefficient of NADH, adjusted for dilution and assay volume. ODt1 represents the optical density one minute post-addition of leucine dehydrogenase, ODt0 denotes the optical density prior to enzyme addition, ε signifies the extinction coefficient of NADH (6.22), 1000 facilitates the conversion from millimolar to micromolar units, 0.3 indicates the assay volume in milliliters, and 0.01 refers to the sample volume in milliliters. During internal assay testing of unknown samples, we consistently compare the variation in optical density of a known standard (500 μM leucine) with the computed value at regular intervals (roughly hourly).
Plasma is obtained by collecting 20 ml of blood in heparinized vacutainers. The blood is centrifuged at 3000 g for 10 minutes, and the plasma is divided into 1-milliliter aliquots. The plasma is augmented by the addition of 0, 20, 40, 60, or 80 nmol each of leucine, isoleucine, and valine, resulting in branched-chain amino acid concentrations of the unaltered plasma plus 0, 60, 120, 180, and 240 nmol. Samples are tested in duplicate using both an ion-exchange amino acid analyzer and an enzyme assay. The quantity in the pure plasma is 420 ± 9 μM as determined by the amino acid analyzer and 436 ± 3.5 μM as measured by the enzyme assay. In both experiments, the concentration escalates in direct correlation to the increased spike concentration. Upon deducting the concentration introduced as a spike, the residual concentration remains indistinguishable from that of the pure sample, regardless of the spike concentration applied. Consequently, the enzyme assay is responsive to the overall quantities of branched-chain amino acids in serum. The enzyme assay overestimates concentrations determined by the amino acid analyzer by 23 ± 19 μM (p < 0.05), as assessed by a paired t-test (95% confidence interval, –9.6 to –36.5), resulting in an inaccuracy of approximately 5% at a concentration of 420 μM. The origin of the discrepancy remains ambiguous, either attributable to inaccuracies in the concentrations of internal standards utilized in the tests or to pipetting mistakes. The intraassay variability, assessed using 5 blood samples tested 15 times with the enzyme assay, was 4.1%.
Leucine dehydrogenase possesses four characteristics that make it suitable for a fast bedside assay: (1) The test exhibits significant substrate specificity for branched-chain amino acids, facilitating its use in the analysis of physiological samples including various amino acids and compounds; (2) during the reaction, NAD is reduced stoichiometrically alongside the oxidation of the branched-chain amino acid. The reduction of NAD can be measured using a portable spectrophotometer; (3) the required sample volume is merely 10 μl, making the test appropriate for multiple sampling or for preterm infants. For multiple sampling, we employ a closed system with two syringes on an intravenous line, allowing discarded blood from the catheter to be returned to the patient. This technique prevents the loss of 5 ml of blood to acquire a 50-μl sample; also, the sample turnaround time, from blood collection to result, can be accomplished in under 2 minutes with practice and an effective setup. The real-time data can be utilized to adjust the infusion rate of an amino acid combination to sustain a steady plasma concentration of branched-chain amino acids, analogous to the glucose clamp technique. This assay may be clinically relevant for sustaining plasma branched-chain amino acid levels during physiological research and for monitoring treatment in clinical circumstances such as diabetic ketoacidosis or during total parenteral nutrition.
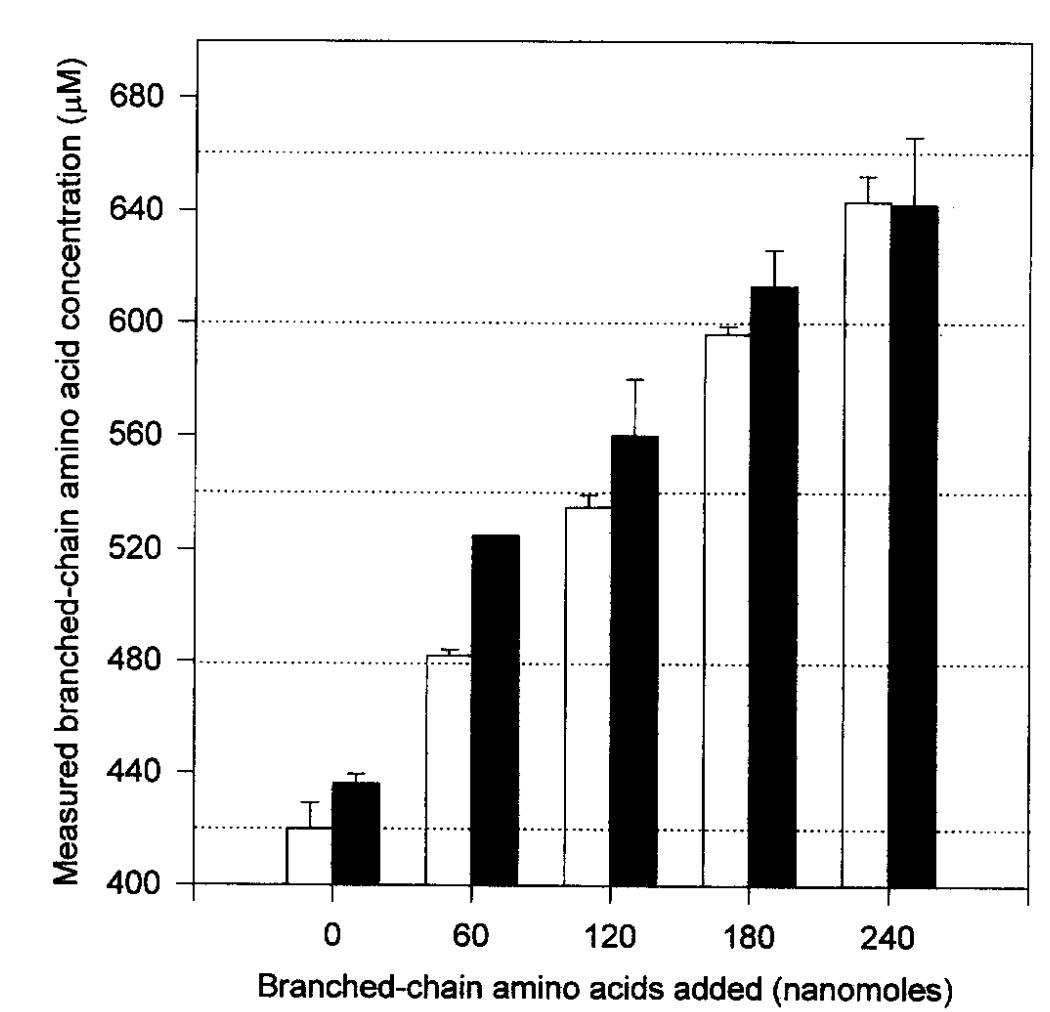 Comparison of BCAA by ion-exchange amino acid analysis and the enzyme assay. (Beckett P R., 2000)
Comparison of BCAA by ion-exchange amino acid analysis and the enzyme assay. (Beckett P R., 2000)
Meat, seafood, dairy, and eggs are the food groups that are rich in BCAAs. For many years, BCAAs have been investigated as potential agents that can increase the production and mass of muscle proteins during exercise, in cases of cachexia (muscle wasting condition), and as we age. Leu is recognized to activate many proteins involved in protein synthesis, including the anabolic signaling molecule mTORC1 (mammalian target of rapamycin complex 1). The potential health benefits of BCAAs in an energy deficit are at odds with the chronic elevations of BCAAs in the blood of people suffering from obesity-related disorders like insulin resistance, type 2 diabetes (T2D), and cardiometabolic disease. In 1969, Cahill's group found that, when comparing the blood of fat people to that of lean people of the same age and sex, they found higher amounts of three BCAAs and the aromatic amino acids phenylalanine (Phe) and tyrosine (Tyr), and lower concentrations of glycine (Gly). Correlations between elevated BCAAs and circulating insulin levels point to insulin resistance as the cause of the increase. Dyslipidemia, an increase in circulating and tissue lipid concentrations linked with obesity, came to the forefront after research suggested that lipids may play a role as a molecular cause of insulin resistance and type 2 diabetes.
It is evident that BCAAs serve as a biomarker for the phenotypes of cardiometabolic diseases. More and more research points to their role in disease development, especially as it relates to obesity. Dietary sugars cause diet-induced obesity by activating ChREBP, which in turn increases the BDK/PPM1K ratio, which in turn suppresses BCAA catabolism, activates DNL, and suppresses FAO. Dyslipidemia is a risk factor for cardiometabolic illnesses, and BCAAs are a biomarker for it since BDK and PPM1K control ACL. Obese rats can have their disease symptoms reversed just by reducing the BDK/PPM1K ratio, either chemically or pharmaceutically. In addition, metabolic health is affected by the addition of BCAAs to obesogenic diets but not low-fat diets, or by limiting their consumption in the diets of obese rats. Lastly, it is consistent with a causal role for BCAAs in T2D pathogenesis that genetic polymorphisms that enhance BCAA levels are strongly associated with risk of future T2D. Cardiometabolic illness pathogenesis is an emerging area that requires and will benefit from more research into the processes by which BCAAs and associated metabolites influence it.
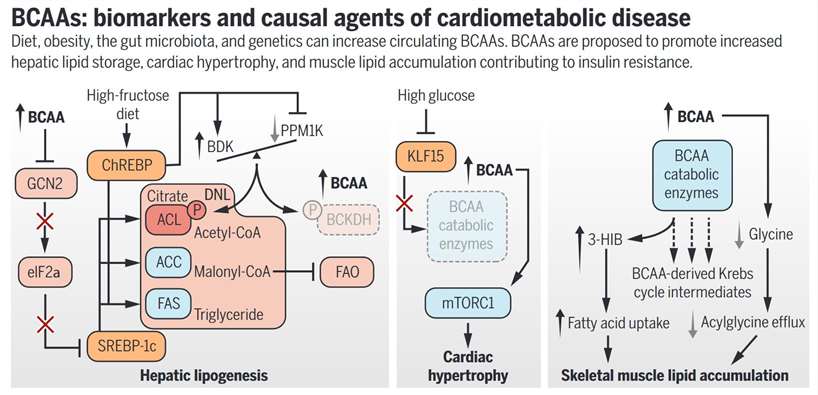 BCAAs: biomarkers and causal agents of cardiometabolic disease. (White P J., et al., 2019)
BCAAs: biomarkers and causal agents of cardiometabolic disease. (White P J., et al., 2019)
Human and animal gastrointestinal illnesses, such as diarrhea and inflammatory bowel disease (IBD), have been the primary focus of AAs as treatments. Obesity, type 2 diabetes, inflammatory bowel disease (IBD), atherosclerosis, colon cancer, and other serious health problems are all linked to a breakdown in intestinal homeostasis, which occurs in this vital organ. The immune system's activity is strongest in the intestines. Accumulating evidence from both animal and human studies suggests that AAs play important roles in regulating intestinal health, in addition to their roles as substrates for protein synthesis and other nitrogenous chemicals, and in the maintenance and growth of organisms. Intestinal mucosal barrier activities, cell migration, anti-oxidative responses, and tight junction integrity could all be enhanced by amino acids like glutamine, arginine, and threonine. Additionally, BCAAs boost gut health by regulating nutrient transporters, immune-related function, and intestinal growth. The majority of research, however, has ignored the intestinal Val and Ile roles in favor of Leu. Leu supplementation has several benefits for the gut, including improving the tight junction in fish and increasing the height of the villus and small intestine in pigs. However, it inhibits intestinal growth at levels as high as 2.57%. Leu supplementation improves intestinal growth by activating mTOR and its downstream pathway, as indicated by the increased mRNA expressions of p70S6K and mTOR in the jejunum and ileum as dietary Leu levels increased from 1.37% to 2.17%. When it comes to fiber and other nutrients, AAs have the ability to influence the gut microbiota, which is crucial for the host's overall health. Modulating the development of insulin resistance and type 2 diabetes can be achieved through the impact of elevated systemic quantities of specific amino acids produced by gut bacteria, including BCAAs. Supplementing the diet of young Jian carp (Cyprinus carpio var. Jian) with ile enhances the bacteria community in their intestines. A linear response was observed in the Lactobacillus count with graded Ile levels, while a quadratic reaction was observed in the populations of Bacillus, Aeromonas, and E. coli. The levels of Aeromonas and E. coli were lowest in fish fed the 11.9 g/kg Ile diet, whereas the largest counts of Intestinal Bacillus were observed in fish fed the 13.9 g/kg Ile diet.
There is a close relationship between BCAAs and other intestinal processes, in addition to their regulation of intestinal development and the expression of intestinal AAs transporters. By influencing the production of endogenous defensin, BCAAs have the potential to improve intestinal health and disease resistance in children and young animals. The group led by Ren examined how BCAAs affected the mRNA expression of β-defensins in porcine epithelial cells. Weaned pigs treated with BCAAs had a rise in β-defensin expression in their jejunum and ileum, and IPEC-J2 cells showed a comparable increase in β-defensin expression after BCAA therapy. Furthermore, the substantial correlation between BCAAs and intestinal function is supported by the high levels of enzymes involved in BCAA metabolism, such as BCAT and BCKD, in the intestine.
Lactation is associated with a substantial increase in whole-body BCAA catabolism as compared to non-lactating individuals. Increased milk supply for nursing newborns may be an effect of BCAA catabolism on the mammary gland's glutamate, glutamine, aspartate, alanine, and asparagine synthesis. Also, the production of glutamate and aspartate in breast tissue is restricted by the main leucine transporter LAT1. At the same time, during lactation, there is an increase in the activity of BCAT and BCKD, two essential enzymes in BCAA catabolism. This increase can be due to a decrease in insulin and growth hormone or an increase in cortisol and glucagon.
There is mounting evidence that bodes well for the relationship between BCAA, milk supply, and the performance of neonatal piglets. Nutritional supplementation with BCAAs enhanced milk protein production but had no effect on milk yield in a sow trial (Control group: 16% CP vs. Treatment group: 23% CP + BCAA). Omitting leucine or BCAA during early lactation in dairy cows with fistulas reduced protein output by approximately 12% and 21%, respectively, when compared to cows having abomasally injected EAA. Research has shown that isoleucine and valine concentrations in milk can be enhanced by shifting the overall dietary Val:Lys ratio from 0.84:1 to 0.99:1. Based on these findings, the proportion of protein in milk is highly dependent on the dietary levels of BCAA, particularly leucine. Insufficient BCAA levels hinder milk protein production because mTORC1-mediated up-regulation of eIF2Bε and eIF2α abundance is deactivated. Nevertheless, a different 2 × 2 × 2 factorial experiment with sows examined the effects of supplementing with valine (0.80 and 1.20%), isoleucine (0.68 and 1.08%), and leucine (1.57 and 1.97%) on milk nitrogen levels. The results showed that leucine and isoleucine did not have any effect. This study highlighted the importance of raising the valine level in sows' diets from 0.8% to 1.2% in order to increase the litter weaning weight of neonatal piglets. The mammary cells may be able to regulate the effect of BCAA on milk production. Among the many potential benefits of BCAA for mammary epithelial cells is an increase in their lifespan, improved functional differentiation, and accelerated growth and proliferation. When administered to bovine mammary cells, leucine and isoleucine increased fractional protein synthesis rates through mTOR and rpS6 phosphorylation, or mTOR, S6K1, and rpS6 respectively, while reducing proteasome protein abundance, ubiquitinated protein levels, and protein degradation rates.
Nutrition during pregnancy is crucial for the growth and development of the fetus and embryos. Changes in foetal health are significantly linked to the onset of chronic disorders in adulthood. There is mounting evidence that BCAA play an essential role in embryonic development. Women whose unborn children have intrauterine growth retardation (IUGR) tend to have lower levels of BCAA in their blood in the umbilical vein and artery than women whose unborn children do not have IUGR. Early embryonic development relies on protein synthesis. Leucine, more than any other BCAA, activates the mTOR signaling pathway, which in turn promotes protein synthesis in various tissues, including skeletal muscle. In order to alleviate IUGR syndrome caused by a low-protein maternal diet, BCAA supplementation (1.8% L-Leu, 1.2% L-Val, and 1% L-Ile) activates the mTOR signaling pathway. In addition, there is evidence that a diet rich in BCAAs can enhance fetal liver IGF-1 and IGF-2 gene and protein expression, which may alleviate fetal growth restriction. On the other hand, another research indicated that oral administration of L-leucine (at doses of 300 or 1,000 mg/kg body weight) during organogenesis had no effect on the pregnancy outcome. with rodents. Diverse dietary leucine concentrations and BCAA combinations may account for the discrepancy in findings. Through its effects on protein synthesis and hormone production, leucine clearly up-regulates foetal growth.
Amino acids not only promote blastocyst quality, which in turn improves embryo implantation, but they are also essential for embryonic development. Because AA are essential for embryonic development, researchers have discovered that they are required to cultivate mouse embryos in vitro. Amino acids inhibit trophectoderm motility and mTOR signaling pathway activation, which leads to embryo implantation in mice. The fact that leucine activates the mTOR signaling pathway suggests that it may have a role in blastocyst formation, one of its most important functions. Blastocyst activation is induced by up-regulation of the leucine transporter SLC6A14, according to recent reports. The available information strongly suggests that leucine plays a crucial role in the development of blastocysts.
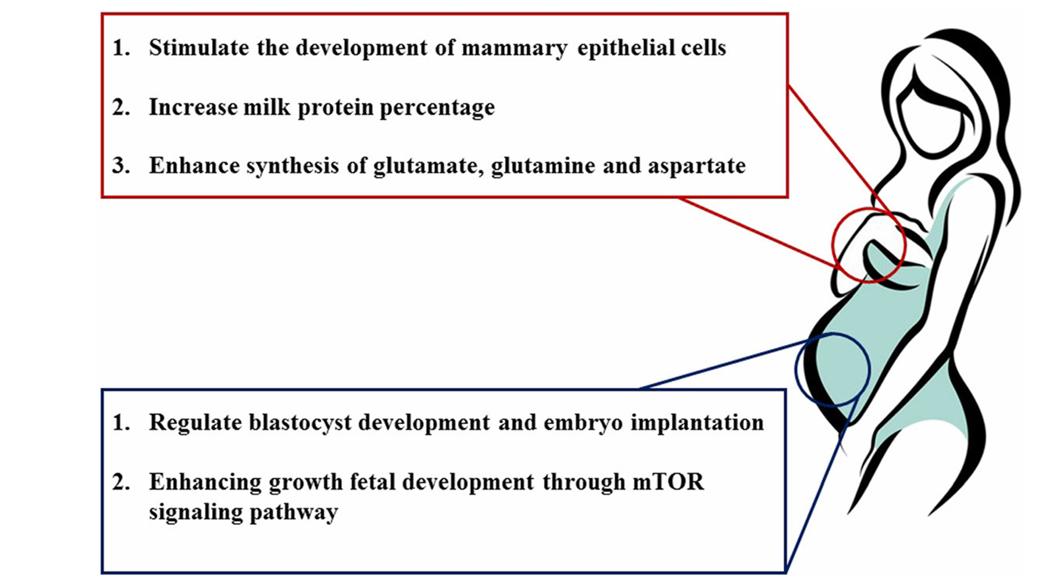 Branched chain amino acids regulate mammary function and embryo development. (Zhang S., et al., 2017)
Branched chain amino acids regulate mammary function and embryo development. (Zhang S., et al., 2017)
References