Amino acids are building blocks of proteins, and the only way to get them is to eat protein. We can classify the amino acids found in proteins as either "essential" or "non-essential" for your health. The human body is unable to produce any of the amino acids that are considered essential. In addition, there are a few amino acids that are not normally needed in the diet but must be provided externally when the body is unable to synthesize them adequately: arginine, cysteine, glycine, glutamine, proline, serine, and tyrosine. According to Dewey's study, the amount of amino acids needed for net protein deposition is a small fraction of the total amino acid requirement in humans. Even in infants, more than 90% of the amino acid demand is associated with maintaining body protein storage.
To this day, essential amino acids (EAAs) are considered essential if either their carbon skeletons cannot be produced de novo by animal cells or if their de novo synthesis is inadequate in comparison to metabolic demands. Cys, His, Ile, Leu, Lys, Met, Phe, Thr, Trp, Tyr, and Val are the EAAs for all mammals. This includes humans, fish, dogs, goats, mice, pigs, rats, shrimp, and dogs. Since the small intestine mucosa does not contain the enzyme pyrroline-5-carboxylate synthase, arginine is an essential amino acid (EAA) for birds and certain mammals (such as cats and ferrets). Even though adults and rats don't need arginine in their diets to keep their nitrogen levels stable, going arginine-free for 9 days drastically reduces spermatogenesis and sperm viability (by as much as 90%, for example). Dietary requirements for both proteinogenic (like arginine) and nonproteinogenic (like taurine in cats, fish, and human infants) amino acids should be defined according to functional needs (such as optimal growth, fertility, lactation, blood circulation, connective tissue health, and immune response). Birds don't have pyrroline-5-carboxylate synthase, thus they can't make proline from glutamate and glutamine, and their poor arginase activity means they can't make proline from arginine either. Poultry and young pigs require nutritional glycine for optimal growth because their glycine utilization rate is significantly higher than their glycine production rate. There is data that suggests a number of fish species are unable to synthesize arginine, and it is also possible that they cannot synthesize enough glycine and proline. Because AA synthesis differs between species, the exact set of EAAs can range from one animal to the next.
Proteins such as glutamine, proline, glycine, taurine, glutamic acid, ornithine, carnitine, c-aminobutyric acid, citrulline, glutathione, and cysteine can be produced by humans from their own carbon, nitrogen, and energy sources. On the other hand, nine amino acids can't be made by humans due to metabolic constraints; thus, they need to be consumed every day. Methionine, histidine, lysine, valine, phenylalanine, tryptophan, isoleucine, threonine, and leucine are all considered essential amino acids. Plants continue to make up a large part of the human diet, even though most essential amino acids can be obtained from animal sources. In fact, for many people in developing nations, plants remain the primary source of nutrition. Although plants produce their own amino acids, plant-based diets, particularly those high in cereals and legumes, are deficient in a number of important amino acids, including threonine, lysine, tryptophan, and methionine. Consequently, agricultural plants have long been a target for the purpose of enriching plant-based diets with certain amino acids.
While alpha-ketoglutarate is present, branched-chain amino acids can be transaminated to produce glutamate and their corresponding alpha-ketoacids, such as alpha-ketoisocaproate from leucine, alpha-keto-beta-methyl-valerate from isoleucine, and alpha-ketoisovalerate from valine. Thereafter, a ketoacid dehydrogenase (BCKAD) oxidatively decarboxylates each of these ketoacids. Further metabolic processing of decarboxylation byproducts results in the production of acetyl-CoA, acetoacetic acid, propionyl-CoA, and succinyl CoA. The fact that branched-chain amino acids can compete for membrane transport with other amino acids, especially tryptophan and tyrosine, is pretty well established. Although branched-chain amino acids do not directly contribute to the production of neurotransmitters, they do influence the transport of other amino acids across the blood-brain barrier and, consequently, the concentrations of some neurotransmitters in the central nervous system.
Histidine is prevalent in hemoglobin. Histidine serves as a precursor for glutamate and histamine. This chemical is implicated in allergic and inflammatory responses.
Lysine serves as a precursor for acetoacetyl-CoA, carnitine, and cadaverine. Carnitine is a chemical that plays a crucial role in intermediate metabolism. Carnitine facilitates the transfer of long-chain fatty acids from the cytosol to the mitochondrial matrix, where beta-oxidation transpires. Carnitine in the body primarily derives from food sources, whereas endogenous synthesis contributes somewhat. Carnitine is primarily produced in the liver from lysine and methionine. Cadaverine is a polyamine synthesized by the intestinal microbiota.
Methionine serves as a precursor for succinyl-CoA, homocysteine, cysteine, creatine, and carnitine. Moreover, it serves as a precursor for the production of S-adenosyl-methionine, which plays a role in the metabolism of polyamines, creatine, and phosphatidylcholine, a membrane phospholipid.
Phenylalanine serves as a precursor for the production of tyrosine and acetoacetyl-CoA. Threonine serves as a precursor for the production of glycine and acetyl-CoA. This amino acid is highly prevalent in mucins.
Tryptophan is a building block for many compounds, such as the B vitamin niacin, the hormone melatonin, the neurotransmitter serotonine (5-hydroxytryptamine), and tryptamine. Another important role for tryptophan is in the production of acetyl-CoA.
 Essential amino acids for human. (EMERY P W., 2005)
Essential amino acids for human. (EMERY P W., 2005)
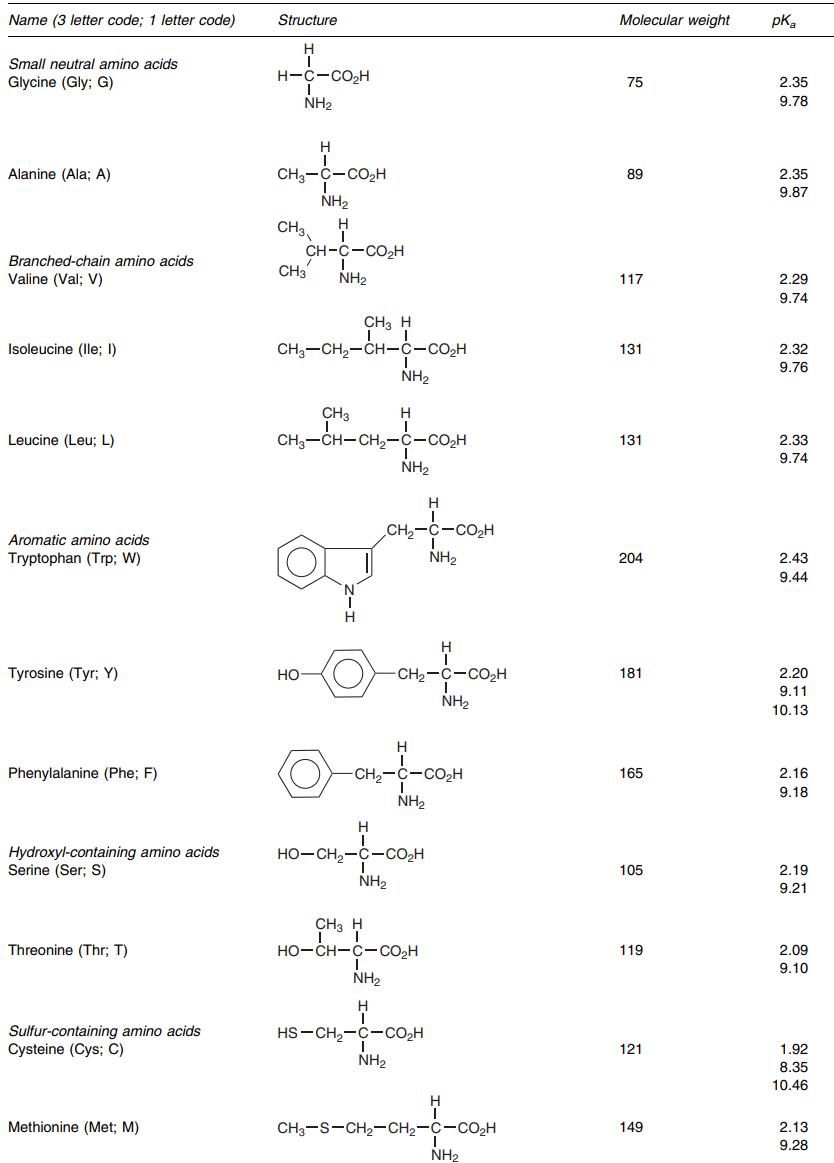 Structures of all amino acids (list 1). (EMERY P W., 2005)
Structures of all amino acids (list 1). (EMERY P W., 2005)
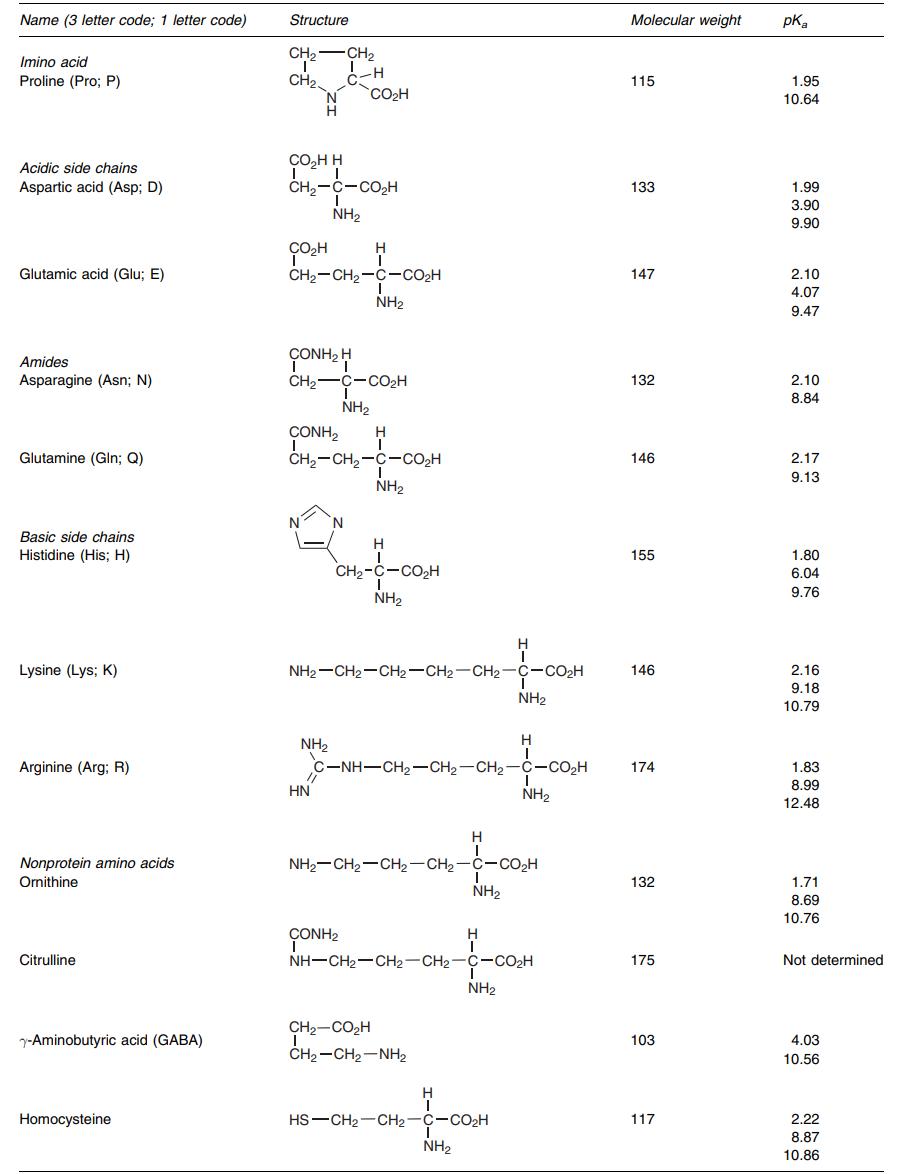 Structures of all amino acids (list 2). (EMERY P W., 2005)
Structures of all amino acids (list 2). (EMERY P W., 2005)
Essential amino acids are not synthesized by the human body in sufficient amounts (or at all). This is why they must be obtained from the diet. Many essential amino acids are synthesized by bacteria, fungi, and plants through metabolic pathways. Shikimate pathway produces aromatic amino acids like phenylalanine and tryptophan. Plants have the ability to synthesize all amino acids, including the essential ones, using nitrogen from the soil (absorbed as nitrate or ammonium) and carbon from photosynthesis.
Cereals and legumes contain the lowest amounts of lysine, making it the most limiting necessary amino acid. Consequently, there have been significant endeavors to enhance the Lys content in plants, particularly seeds, as they constitute the primary sources of food for humans and feed for livestock in underdeveloped nations. Aspartate kinase (AK), the initial enzyme of the Asp family pathway, and the feedback-insensitive dihydrodipicolinate synthase (DHPS), the initial enzyme specifically involved in lysine synthesis, are the two primary enzymes that regulate the lysine pathway. Nevertheless, the catabolism of lysine into its component parts is an efficient process that begins with the bi-functional enzyme lysine-ketoglutarate reductase (LKR)/saccharopine dehydrogenase (SDH) and continues through the tricarboxylic (TCA) cycle. Our knowledge of plant lysine metabolism goes back to the 1960s, when the opaque-2(o2) mutant, a maize variety with an elevated lysine content but a decreased amount of lysine seed storage proteins (zeins), was first identified. Due to the extensive knowledge of lysine biosynthesis, some content improvement efforts focused on either expressing feedback-insensitive DHPS or avoiding lysine degradation into the TCA cycle.
Three more essential amino acids—Thr, Met, and Ile—are produced by a different branch of the Asp family biosynthetic route; they too are vital for plant development and human nutrition. Two important enzymes in the aspartate pathway, homoserine dehydrogenase (HSD) and aspartate kinase (AK), can be found in plants as mono- or bifunctional proteins that are controlled by Thr feedback. The catabolism of Thr also controls its level. Met is synthesized from O-phosphohomoserine, an intermediary of the Asp route. Furthermore, Met's catabolic enzymes control its level as well. In instance, diminished disease resistance is one of the nonspecific symptoms of protein shortages in humans caused by Met's scarcity in cereal and legume crops.
Pyruvate is the first step in the biosynthetic pathway for Val, while 3-methyl-2-oxobutanoate is the first step for Leu. Acetohydroxyacid synthase (AHAS), ketol acid reductoisomerase (KARI), dihydroxyacid dehydratase (DHAD), and branched-chain aminotransferase (BCAT) are the four enzymes involved in the production of Val and Leu. Similar to how drought, intense light, and heat stress all increase Val and Leu levels, environmental disturbances often do the same. Aromatic amino acids (AAAs) like as tyrosine and phenylalanine are not only necessary for protein biosynthesis but also serve as building blocks for a wide range of plant-based compounds, including pigments, hormones, and alkaloids. Phe is a common precursor of various phenolic compounds, including flavonoids, condensed tannins, and phenylpropanoid/benzenoid volatiles, while Trp is a precursor of alkaloids, phytoalexins, and auxin, the plant hormone. Phosphoenolpyruvate and erythrose 4-phosphate are converted to chorismate via the shikimate pathway, which is the source of all AAAs. Trp and Phe are synthesized by distinct postchorismate processes. Animals lack these routes, whereas fungus, plants, and microbes all have them. Plants utilized as food for humans and animal feed should, therefore, have a higher concentration of these AAAs.
Studies on plant histidine biosynthesis have lagged much behind those on fungal and bacterial systems. There are nine enzymes in the histidine biosynthetic pathway; however, there is mounting evidence that ATP-phosphorilbosyl transferase plays a pivotal role in controlling histidine biosynthesis.
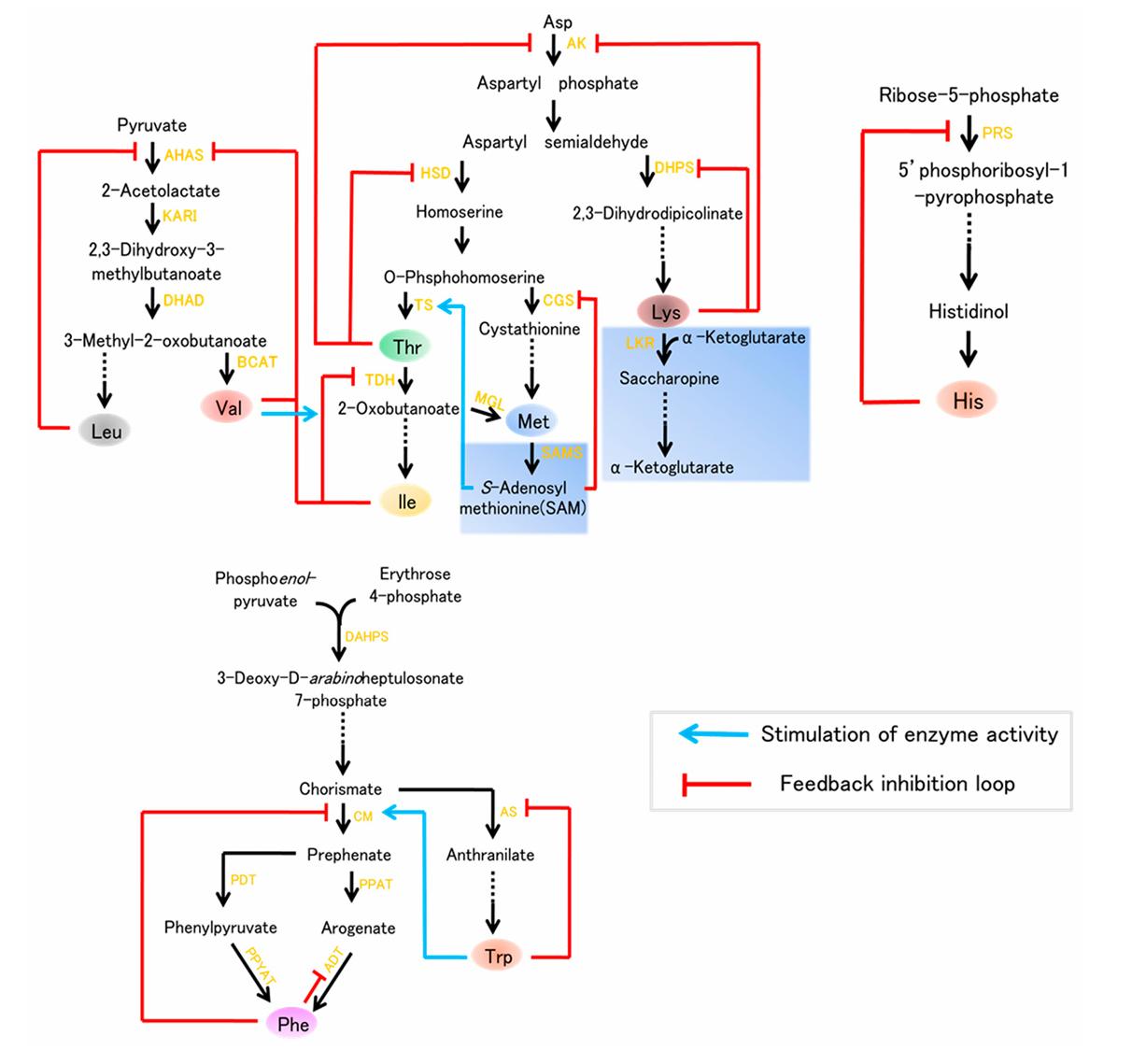 Nine essential amino acids biosynthesis. (Wang G., et al., 2017)
Nine essential amino acids biosynthesis. (Wang G., et al., 2017)
Inadequate dietary intake of EAAs causes deficiencies in humans and other animals. boosting the consumption of amino acids or protein compensates for an EAA deficiency by boosting the oxidation of other amino acids. This occurs because all extra AAs are broken down in a tissue-specific way due to the limited availability of this EAA, which in turn inhibits the use of other AAs for protein synthesis. Insomnia, irritability, low appetite, vomiting, impaired nutrient absorption, storage, and transportation, decreased neurotransmitter synthesis, emotional disorders (such as moodiness, severe depression, and anxiety), impaired oxygen transport, anemia, and impaired appetite are all symptoms of EAA deficiencies. In addition to lower levels of albumin and AAs in plasma, affected individuals display an endocrine imbalance with lower levels of insulin, growth hormone, insulin-like growth factor I, and thyroid hormones in plasma. Young people also experience stunted growth and impaired development, including cognitive development. Overall, the body expends less energy and stores more white fat, and there is wasting of skeletal muscles, fatigue, and weakness. Further complications that can arise from insufficient consumption of EAAs include libido loss, impaired spermatogenesis, decreased fertility, and embryonic death. Intrauterine growth restriction also leads to decreased milk production and has long-lasting negative effects on postnatal growth, metabolism, and health, including an increased risk of obesity, infections, and cardiovascular abnormalities. In addition, those who lack EAA experience a host of health problems, such as accelerated aging, increased oxidative stress, impaired antioxidative reactions, headaches, dizziness, impaired immune response, infections, and higher rates of infection-related illness morbidity and death, heart failure, cardiovascular abnormalities, hypertension, and tissue fluid retention, which manifests as periorbital and peripheral edema, swelling in the legs, feet, and abdomen, and cardiovascular abnormalities. Lastly, these individuals display several symptoms, including a decrease in calcium and bone density, irregularities in the teeth, hair thinning or loss, a decrease in pigment production, the appearance of gray hair, skin that is pale, dry, flaky, or atrophying, and a generalized loss of hair and scalp density. Dietary protein deficiency leads to poor growth, cardiovascular dysfunction, and an increased risk of infectious diseases. It also worsens metabolic profiles (such as dyslipidemia and hyperglycemia), increases the deficiency of other nutrients (such as iron and vitamin A), and ultimately causes death in subjects.
The slow rate of protein synthesis in cells and tissues, especially skeletal muscle, is the fundamental cause of the severe difficulties experienced by humans and other animals with an EAA shortage. Proteins are essential for many biological processes, including: 1) nutrient digestion and absorption in the small intestine; 2) transport of nutrients like iron, cholesterol, and long-chain fatty acids (FAs) into the bloodstream; and 3) nutrient oxidation, which involves converting glucose and FAs to carbon dioxide and water. So, in underdeveloped parts of the globe, people still struggle to get enough of some micronutrients and EAAs, such as vitamin A, iron, zinc, and folate. A deficiency in at least one EAA affects over half of the elderly who are confined to their homes in the US. By influencing processes involving foetal and neonatal programming, inadequate EAA consumption during pregnancy and the postnatal period has far-reaching negative effects on children. Not only does this nutritional condition have a negative impact on fetal and newborn growth, but it also increases the risk of metabolic syndrome (which includes diabetes, hypertension, and obesity) and lowers adult quality of life. In particular, stunting has devastating societal and human health consequences, impacting not just the physical strength of affected individuals but also their reproductive health and subsequent generations. Sarcopenia and impaired skeletal-muscle function are both made worse by EAA deficiency in the elderly. All animals, whether domestic, wild, or companion, face the same challenges.
Essential amino acids (EAAs) comprise nine amino acids that the body cannot synthesize and must obtain from dietary sources, including histidine, lysine, methionine, and tryptophan, among others. Branched-chain amino acids (BCAAs) are amino acids distinguished by an aliphatic side chain that features a core carbon atom connected to three or more carbon atoms. Among the proteinogenic amino acids, three are classified as branched-chain amino acids: leucine, isoleucine, and valine. The three proteinogenic BCAAs are categorized as essential amino acids (EAAs) for humans, constituting 35% of the essential amino acids in muscle proteins and 40% of the preformed amino acids required by mammals. Essential amino acids (EAAs) are vital for protein synthesis, enzymatic activity, and hormone production, whereas BCAAs particularly augment muscle metabolism, energy production, and recovery. Unlike other essential amino acids, branched-chain amino acids are metabolized directly in muscle tissue rather than the liver, making them preferred in sports nutrition. All BCAAs are EAAs, however not all essential amino acids are branched-chain amino acids.
Among the approximately twenty amino acids that are utilized in the construction of protein, the body is unable to synthesize the necessary amino acids, which results in an absolute demand for the consumption of these amino acids through the food. On the other hand, amino acids that are not essential to the body's functioning can be easily synthesized in large quantities within the human body. Last but not least, there are certain amino acids that are regarded as conditionally necessary. This indicates that the body is able to create sufficient quantities of these amino acids when the physiological conditions are normal. The synthesis rates of these amino acids, on the other hand, will become insufficient if the body is subjected to situations that are physiologically challenging or stressful. In this regard, the amino acids histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine are considered to be indispensable or essential. The optimization of human performance involves a significant amount of emphasis on the equilibrium of proteins that are present within skeletal muscle. It is important to note that studies have shown that there is an absolute requirement for the essential amino acids in order to maximize the synthesis of skeletal muscle proteins.
 Essential and nonessential amino acids in cancer cells. (Bröer S., 2020)
Essential and nonessential amino acids in cancer cells. (Bröer S., 2020)
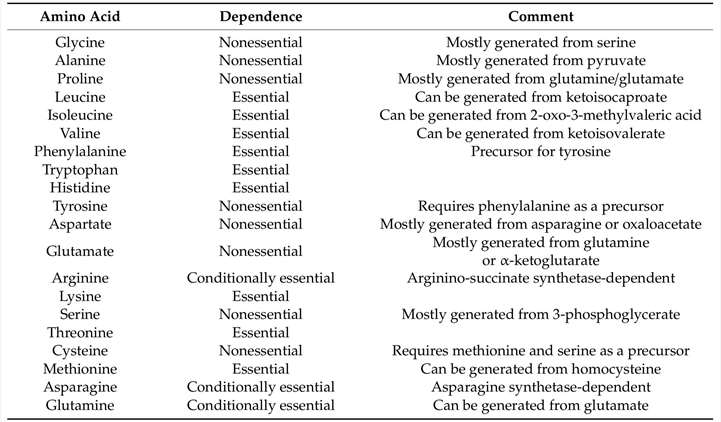
Multiple studies have indicated that the deprivation or restriction of dietary EAAs leads to significant abnormalities in energy balance, culminating in notable changes in fat mass and body weight. Researchers have observed that animals deprived of an EAA experienced weight loss long ago. The degree of weight loss in rats varies with the exclusion of specific amino acids. In these preliminary studies, researchers have mostly concentrated on the impact of EAA deficiency on protein metabolism. Over the past two decades, the significance of amino acids in the regulation of energy homeostasis has been evident. This review examines the research concerning the impact of essential amino acid shortage or limitation on body weight and energy balance. The absence of any individual EAA in the diet is recognized to diminish fat mass and body weight in rodents. Diets lacking BCAA have garnered significant interest, as multiple studies indicate elevated circulating BCAA levels in obese human and animal models. Considering that the total absence of a single EAA results in detrimental long-term health consequences, researchers have explored the ideal concentration of dietary EAAs that can facilitate weight reduction without inducing significant deleterious effects. Thus far, the majority of research on the dietary limitation of an essential amino acid has been on methionine. The consequences of dietary methionine limitation are well documented. The initial research on methionine restriction commenced with a 1993 report indicating that a diet low in methionine (0.17% of the diet [w/w] versus 0.86% in controls) extended the lifespan of rats. Evidence indicates that, besides prolonging lifespan, 80% metabolic restriction reduces fat mass and body weight while inducing hyperphagia. The consumption of an 80% Met restricted diet for either short-term (4-12 weeks) or long-term (80 weeks) may provide the aforementioned effects in growing, adult, or aged rats. Although the majority of initial research on dietary methionine restriction was conducted in rats, later studies including mice have demonstrated that the responses to methionine restriction are almost identical in all significant aspects.
 Functions of essential amino acid deprivation or restriction on energy balance. (Xiao F., et al., 2022)
Functions of essential amino acid deprivation or restriction on energy balance. (Xiao F., et al., 2022)
Gut microbiota, or gut flora, refers to the intricate assemblage of bacteria residing in the digestive systems of humans and animals. In humans, the gut microbiota contains the highest quantity of bacteria and the greatest diversity of species relative to other regions of the body. Obesity correlates with alterations in the composition and diversity of the gut microbiota. The relative abundance of Bacteroidetes is diminished in obese individuals compared to lean individuals, and this abundance increases with weight reduction on a low-calorie diet. Increasing evidence indicates that gut bacteria play a role in regulating host energy balance via modulating lipid, glucose, and protein metabolism. Gut microbiota facilitates energy balance by generating short-chain fatty acids (SCFAs) via colonic fermentation, which entails the anaerobic decomposition of dietary fiber, protein, and peptides. Short-chain fatty acids (SCFAs) serve as energy substrates and modulate gut hormones, including peptide YY (PYY) and glucagon-like peptide-1 (GLP-1), through their receptors to mitigate diet-induced obesity. The gut microbiota has been demonstrated to influence the control of bile acids and cholesterol metabolism in both humans and animals. Firmicutes and Bacteroidetes rapidly participate in the regulation of bile acid modification and oversee bile acid-mediated endocrine processes, including the homeostasis of triglycerides and cholesterol. Moreover, obesity is marked by chronic low-grade inflammation. The synthesis of lipopolysaccharides (LPS), a principal component of the outer membrane of Gram-negative bacteria found in the gut, activates inflammatory cytokine pathways. Antibiotic-induced alterations in gut microbiota may diminish adipose tissue inflammation in mice subjected to a high-fat diet.
Dietary factors significantly influence the modification of gut microbiota in both humans and animals, including amino acids. Dietary methionine limitation enhances gut microbiota and diminishes intestinal permeability and inflammation in high-fat diet mice. Under HFD, the restriction of Met enhances the proliferation of SCFA-producing bacteria such as Bifidobacterium, Lactobacillus, Bacteroides, Roseburia, Coprococcus, and Ruminococcus, as well as inflammation-suppressing bacteria Oscillospira and Corynebacterium. Met restriction also reduces the prevalence of inflammation-inducing bacteria Desulfovibrio in colonic contents. Furthermore, Met restriction enhances intestinal barrier integrity, modulates inflammatory responses, and reduces oxidative stress levels. Nonetheless, it remains uncertain if the gut microbiota is a result or a cause element, as well as whether specific gut microbial species are responsible for the changes in energy balance after EAA shortage or restriction, necessitating further research. The impact of varying diets on food absorption efficiency and its subsequent implications on body weight is mostly unexamined. Furthermore, there is a lack of direct data about changes in food absorption efficiency under essential amino acid deficiency or restriction diets. The gut microbiota is recognized for its significant influence in the efficiency of food absorption. Certain aforementioned microorganisms, like Bifidobacterium and Lactobacillus, are linked to the absorption of certain nutrients from meals, such as fats, calcium, and iron. We hypothesize that food absorption efficiency may vary with dietary methionine limitation. This idea is corroborated by research indicating that dietary methionine limitation diminishes the intestinal absorption of xanthine. Nonetheless, it remains ambiguous whether alterations in microbiota influence overall food absorption efficiency under Met restriction or other dietary regimens, necessitating more investigation.
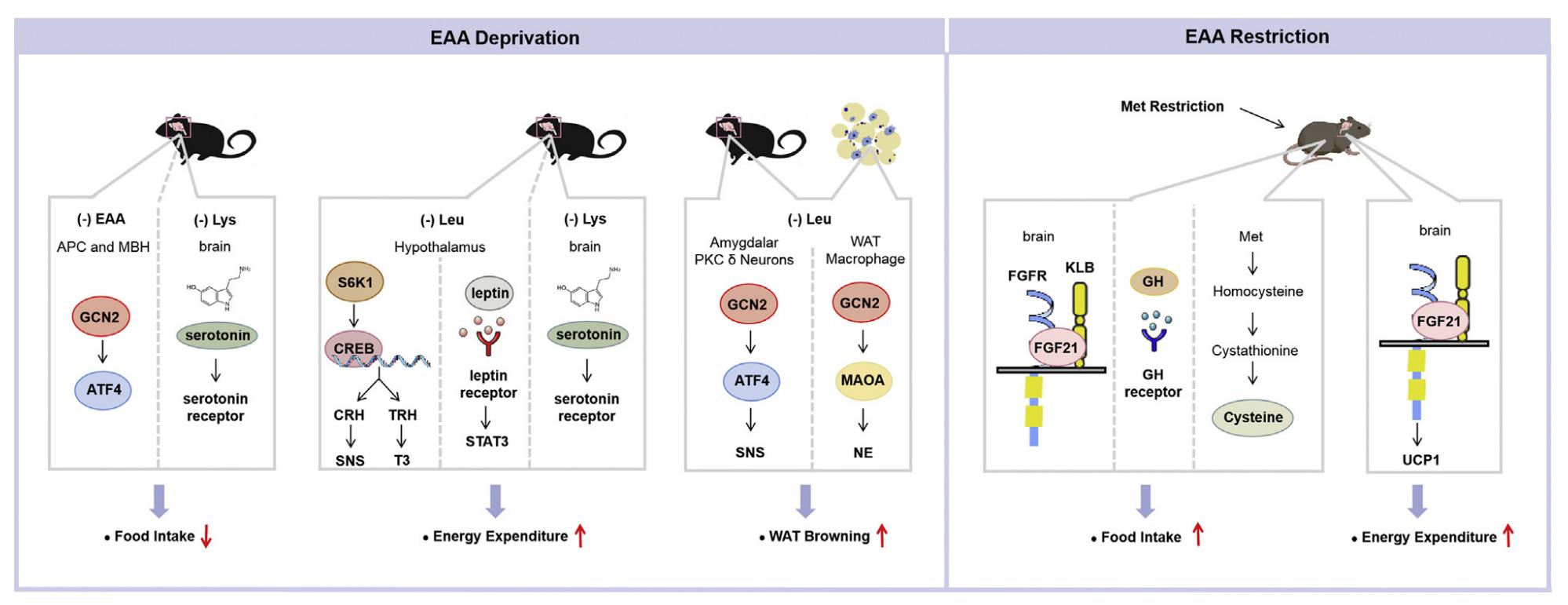
Leucine, a BCAA, is particularly noteworthy in the context of amino acid-induced anabolism due to its exceptional efficacy in promoting protein synthesis via the mTOR pathway in adults. Notably, the BCAAs valine and isoleucine do not exhibit the same anabolic efficacy as leucine, despite their structural similarity. Nonetheless, other amino acids such as glutamine and arginine, which are conditionally essential for babies, seem to significantly influence the regulation of mTOR activity during the initial phases of mammalian development.
The stimulatory effects of amino acids, particularly leucine, on the commencement of protein synthesis have been documented in various tissues, including muscle, heart, liver, pancreas, jejunum, and ovary. The regulating function of EAAs in protein expression occurs during the start stage of mRNA translation, resulting in enhanced synthesis of ribosomal proteins. The stimulatory effect is observed just in the presence of EAAs and is specifically ascribed to the action of BCAAs. Leucine is the most effective in initiating protein synthesis in vitro, and its stimulatory effect on muscle protein synthesis is also observable in vivo, necessitating the presence of either insulin or carbs. Single oral doses of leucine, constituting up to 100% of the daily requirement for this amino acid, have been demonstrated to enhance skeletal muscle protein synthesis in a concentration-dependent way in food-deprived rats; however, the anabolic response to leucine administration varies with age. Older rats exhibit enhanced acute anabolic responses to leucine-enriched diets after 10 days of supplementation, which is maintained for at least 30 days. Unlike aged rats, adult rats consuming comparable leucine-enriched meals exhibited maximally enhanced protein synthesis rates in the postabsorptive state after 10 days. Elevated leucine consumption correlates favorably with the hyperphosphorylation of 4E-BP1, the dissociation of the 4E-BP1-eIF4E complex, the phosphorylation of eIF4G at Ser1108, the interaction of eIF4G with eIF4E, and the phosphorylation of S6K1 at Thr389, thereby facilitating translation initiation in perfused rat hindlimb. Phosphorylation of S6K1 is attenuated after amino acid infusion in older individuals, correlating with a reduced protein synthesis response relative to young adults. Besides initiating translation, leucine and insulin promote elongation by activating (dephosphorylating) eEF2 via a mTOR-mediated mechanism.
 Regulation of skeletal muscle protein synthesis by essential amino acids. (Dillon E L., 2013)
Regulation of skeletal muscle protein synthesis by essential amino acids. (Dillon E L., 2013)
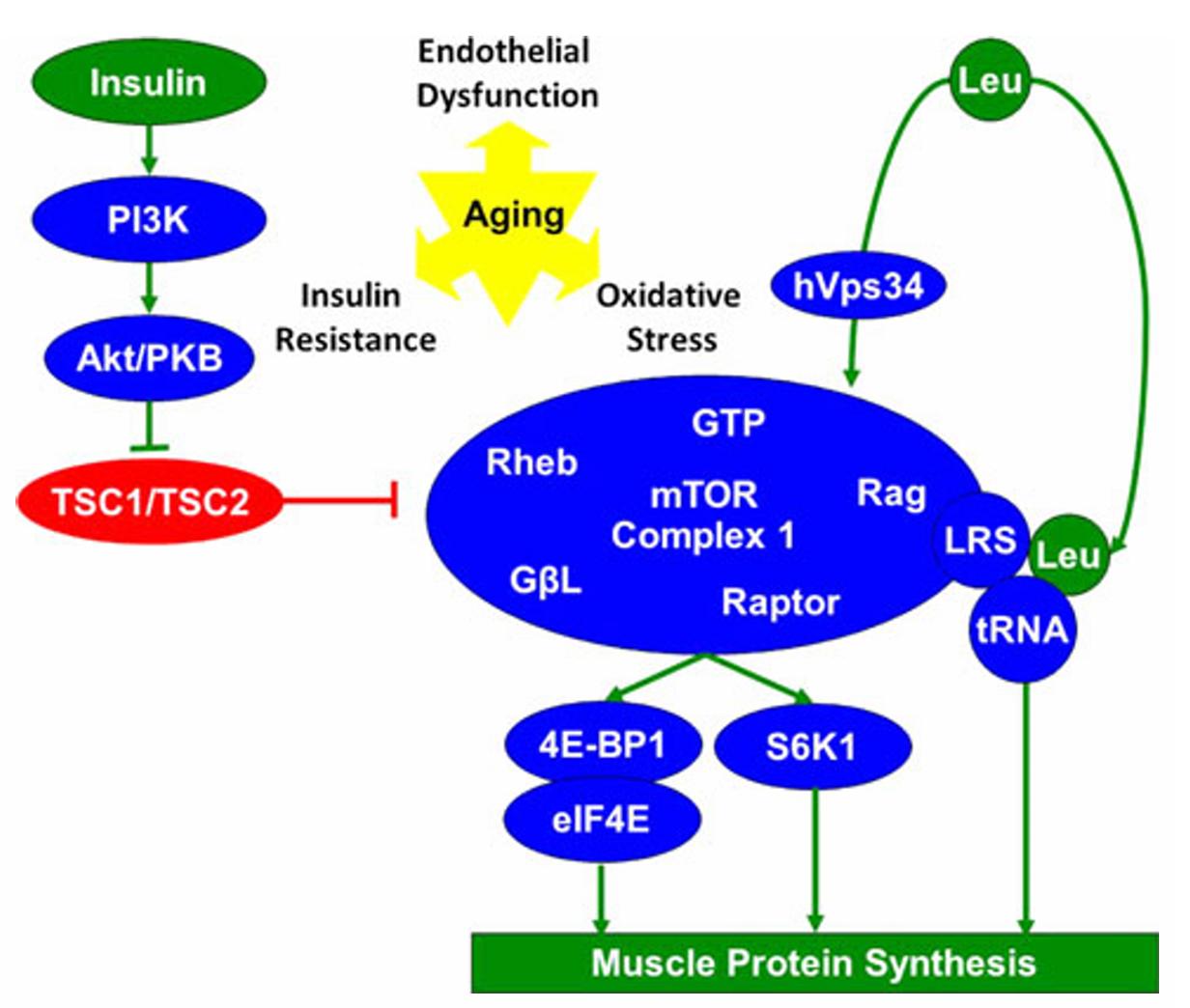
With the exception of Met and Val, supplementation with other essential amino acids has been documented to decrease body weight under specific conditions. Nevertheless, the outcomes are incongruous. Potential factors contributing to these variations may include dietary composition (e.g., fat %), additive dosage, treatment duration, animal strain, and sex. Numerous studies indicate that oral supplementation of Leucine or Isoleucine diminishes fat mass and body weight in mice concurrently subjected to a high-fat diet, but not in already obese mice or those on a standard chow diet. Other researchers did not detect these effects. A potential cause for this could be the various animal strains, which indicate fundamental variances in genetic variants. Oral Leu supplementation may promote weight loss in mice but not in rats under a high-fat diet, indicating species-specific responses to Leu supplementation. The animal's sex may be an additional factor. Although the majority of studies focus on male animals, female animals frequently exhibit distinct responses to dietary problems. Supplementation with a high-fat diet (45% fat) and 1.5% leucine in drinking water reduces belly fat in female mice, but not in male mice. Generally, the effects of EAA supplementation on weight loss may involve reduced food consumption and/or enhanced energy expenditure. Lysine, histidine, and phenylalanine supplementation suppresses food consumption. Excessive leucine consumption does not influence food intake on a high-fat diet, but it does elevate energy expenditure. Moreover, these diets influence thermogenesis and lipid metabolism. Supplementation of leucine, isoleucine, histidine, or threonine enhances UCP1 expression in brown adipose tissue and promotes the browning of white adipose tissue. Supplementation with leucine and threonine was demonstrated to enhance the expression of proteins associated with lipolysis, including phosphorylated hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL).
 Effects of essential amino acid supplementation on energy balance. (Xiao F., et al., 2022)
Effects of essential amino acid supplementation on energy balance. (Xiao F., et al., 2022)
A study examined the impact of a supplement including 9 essential and 3 non-essential amino acids on muscle soreness and injury by comparing two endurance exercise sessions of the elbow flexors with either amino acid or placebo treatment in a double-blind crossover design. The supplement was consumed 30 minutes before to (10 hours post-fasting) and immediately following exercise (Experiment 1), or 30 minutes prior to (2-3 hours post-breakfast), immediately post-exercise, and on eight further occasions throughout a four-day post-exercise period (Experiment 2). Muscle soreness and indications of muscle injury were evaluated across supplement conditions for four days post-exercise using a two-way ANOVA. No notable differences across treatments were seen in Experiment 1; however, plasma creatine kinase, aldolase, myoglobin, and muscular soreness were considerably reduced in the amino acid condition compared to the placebo in Experiment 2. The findings indicate that amino acid supplementation mitigates delayed onset muscle soreness (DOMS) and muscle damage when consumed during recovery days.
A 30-day liquid meal containing or without mixed essential amino acids (given at a dose of 1 g/kg per kg per day) was administered to Sprague-Dawley rats via the enteral route. Using the Langendorff method, hearts from rats that were sedated were perfused and then randomly assigned to one of three groups. While the control group received 60 minutes of buffer perfusion, the groups treated with amino acids or subjected to ischemia-reperfusion were given 35 minutes of ischemia and then either 60 or 120 minutes of reperfusion. The use of amino acid supplements reduced the size of infarcts (22 ± 1.8% vs 33 ± 2.5%; p<0.05) and the incidence of cardiomyocyte apoptosis, as determined by the co-localization of TUNEL and caspase-3-positive staining (p <0.01). Treatment with amino acids over an extended period of time decreased the number of cardiomyocytes showing cleaved caspase-9 immunostaining (p <0.01), but had no effect on caspase-8 processing. Caspase activity testing yielded similar results throughout the entire heart (p <0.01). A significant decrease in mitochondrial release of cytochrome c occurred in tandem with the decreased activation of caspase-9 observed in hearts treated with amino acids. Adenosine triphosphate (ATP) concentration and rate of ATP synthesis in isolated mitochondria were decreased by more than 75% in control hearts following 2 hours of reperfusion (p <0.05 compared to control hearts); in hearts that were given amino acids as a supplement, these values reverted to the control group (p <0.01). Lastly, the rate of oxygen consumption in myocardial skinned bundles was significantly decreased in hearts that were subjected to ischemia-reperfusion control and nearly restored to normal in hearts that were treated with amino acids (about 20% and 93% of the normoxic hearts' values, respectively; p <0.01). Based on these findings, it appears that oral amino acid supplementation reduces the severity of ischemia-reperfusion injury in rats' hearts via preserving the generation of high-energy phosphates by mitochondria.
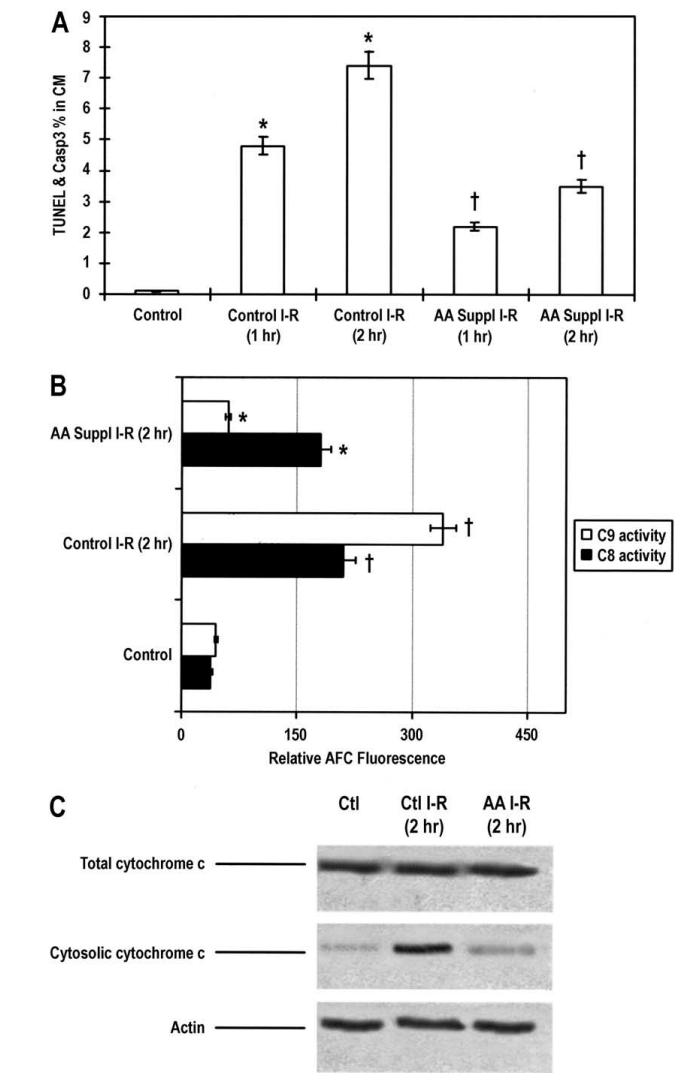 The effects of essential amino acid supplementation. (Scarabelli T M., et al., 2004)
The effects of essential amino acid supplementation. (Scarabelli T M., et al., 2004)
References