Amino acids can be divided into polar and nonpolar groups according to the polarity of the side chains by using this classification system. It is well accepted that polar amino acids have a more powerful impact on CaCO3, particularly negatively charged aspartic acid and glutamic acid. These amino acids have the ability to significantly interact with deprotonated COO− on their side chains and Ca2+ in solution. In light of the fact that nonpolar amino acids are hydrophobic, it is generally accepted that they have a negligible impact on CaCO3 crystallization. The amino acids that are considered nonpolar are those that have side chains that are hydrophobic and do not interact well with water. The majority of the time, these amino acids are located inside the inner of proteins. They make it possible for the structure to remain stable by preventing them from coming into touch with the watery environment. A hydrophobic core that helps to maintain the structure of proteins is often formed by nonpolar amino acids, which are typically buried deep inside the protein's interior. Anchoring transmembrane proteins is accomplished by the interaction of nonpolar residues with the lipid bilayer. Valine (Val), leucine (Leu), and isoleucine (Ile) are examples of branched-chain amino acids, which play an important role in the metabolism of muscles and provide a source of energy while exercising. Both methylation reactions and the production of neurotransmitters need methionine and tryptophan as precursors of their respective processes.
Phenylalanine, glycine, isoleucine, proline, alanine, valine, leucine, methionine, and tryptophan are all examples of nonpolar amino acids. The hydrophobic character of these nonpolar amino acids is shown by the presence of an aliphatic or aromatic group. Valine's side chain is a branched isopropyl group (-CH(CH3)2). Leucine contains a branched isobutyl group (-CH2-CH(CH3)2). Isoleucine has two chiral carbon atoms in its chemical structure. Methionine contains a thioether group (-CH2-CH2-S-CH3), and functions as the start codon in protein synthesis. Proline is an unusual amino acid because it creates cyclic structures by connecting two carbon atoms, rather than having free α amino and carboxyl groups. Along with its alanine group, phenylalanine has a phenyl group. In addition to providing methyl groups for metabolism, amino acids containing sulfur are crucial for protein synthesis.
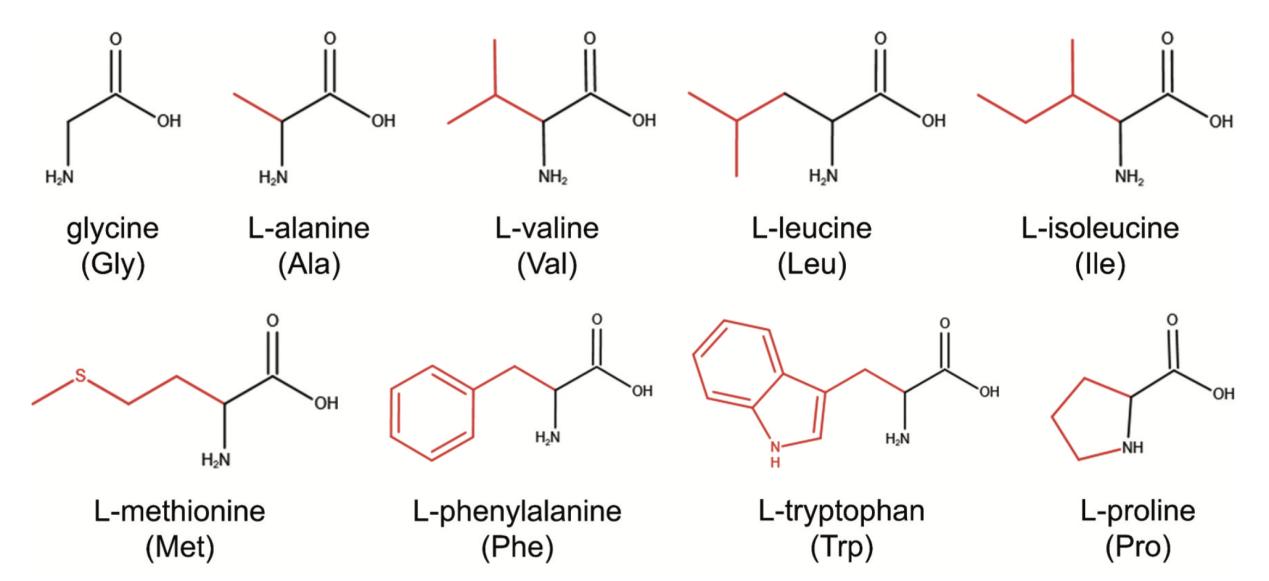 Molecular structures of nonpolar amino acids in levorotatory form. (Zhang K., et al., 2024)
Molecular structures of nonpolar amino acids in levorotatory form. (Zhang K., et al., 2024)
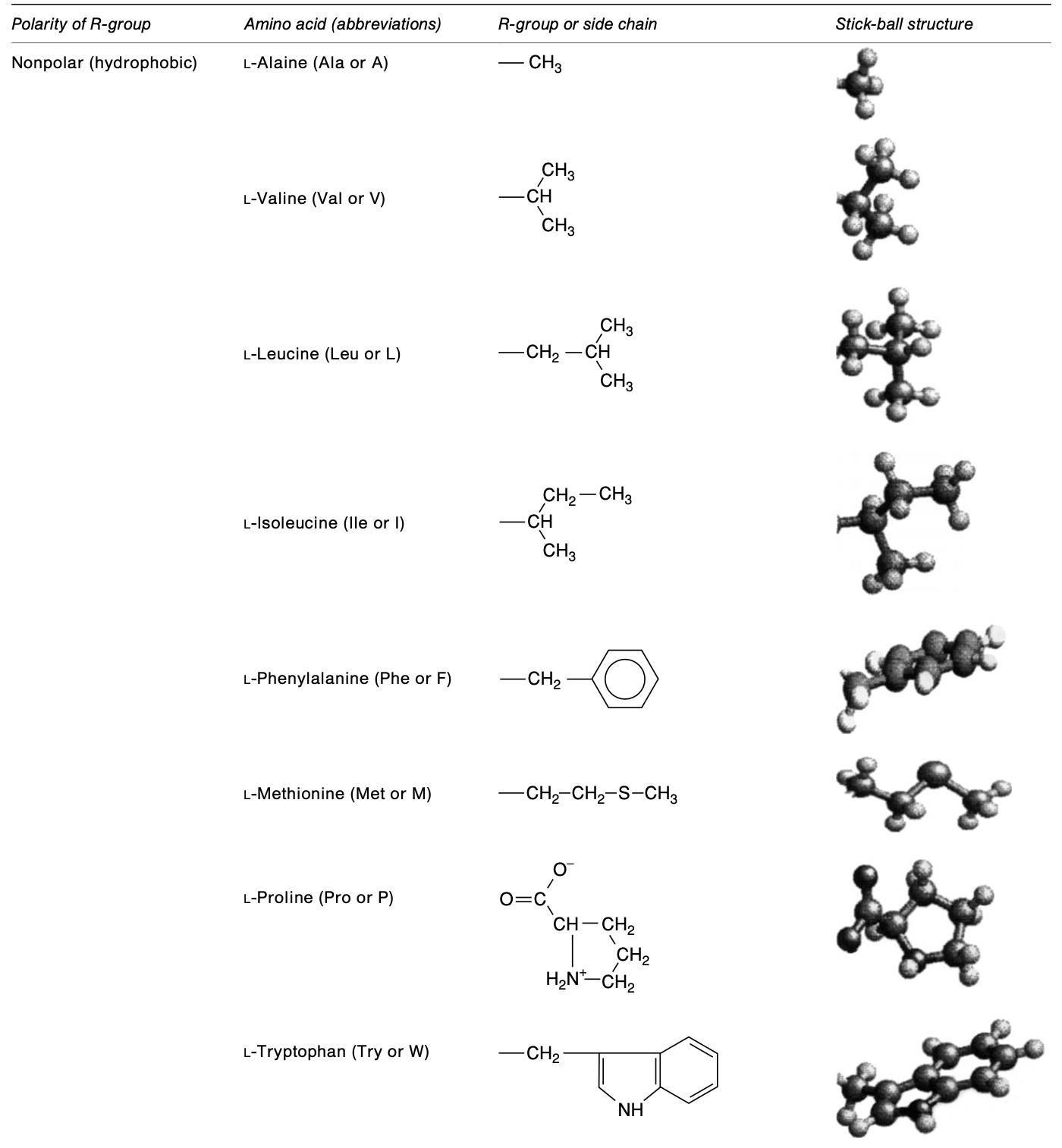 The structure of nonpolar amino acids. (Amaya-Farfan J., et al., 2003)
The structure of nonpolar amino acids. (Amaya-Farfan J., et al., 2003)
Indeed, nonpolar amino acids are hydrophobic, which means that they have a tendency to shun water and other surroundings that are polar. This feature is a result of the fact that their side chains are composed of hydrocarbons or other nonpolar functional groups. These groups do not establish hydrogen bonds and do not interact well with water molecules. There are no polar groups or charged areas included within the side chains of these amino acids, which would allow them to interact with water. There is a mechanism that is driven by the hydrophobic effect that causes the nonpolar side chains to cluster together when they are put in an aqueous (water-based) environment. This is done in order to reduce their interaction with water. The clustering of these proteins is a factor that contributes to their folding. Amino acids that are nonpolar are often buried deep inside proteins, away from the presence of water. Through the formation of a hydrophobic core, this helps to maintain the structure of the protein. As an example, tryptophan is an aromatic amino acid that contains an indole group, which is a fused benzene and pyrrole ring. It is the most complex and biggest amino acid, and it has a somewhat hydrophobic nature. However, owing to the presence of the indole group, it is often classed as a nonpolar amino acid.
There is just one hydrogen atom in the R-group, which makes Glycine the smallest of all the amino acids. The ability of a protein to fold into a compact form is made possible by glycine, which is the smallest residue known to exist. Due to the fact that glycine is present in every third amino acid, collagen is able to fold into a tight triple helix. Collagen is characterized by a consistent sequence that is denoted as [-Gly-Pro-X-]n, where X is often hydroxyproline. The hydrogen atoms that are connected to the α-carbon atom have a negligible impact on the ionization and high degree of polarity that are present in the α-amino/α-carboxylic system. Free glycine is the component that gives shrimp their characteristic sweet flavor. A Greek term that is similar to glucose (glycos) is where its name originates from.
A side chain that is extremely hydrophobic and bulky is found in valine. It is possible for the muscle to metabolize this amino acid, which is classified as a necessary amino acid for humans (the typical adult needs 0.7 grams per day). Valine, leucine, and isoleucine are the amino acids that belong to the branched-chain category.
The leucine side chain is aliphatic, voluminous, and very hydrophobic. It is vital for humans (typical people need 0.98 g/day) and may be digested in muscle tissue. Leucine, isoleucine, and valine constitute the branched-chain amino acids.
The hydrophobicity of this amino acid is rather high. Its four possible isomers are all due to its two asymmetric carbon atoms. In proteins, there is only one isomer. The muscle can digest it, and it is nutritionally required for humans (typical people need 0.7 g/day). Amino acids that form a branching chain include isoleucine, valine, and leucine.
Metathionine, like cysteine, is an amino acid that contains sulfur. Although methionine's sulfur may undergo oxidation, it cannot create disulfide bonds. Because of its R-group, methionine is very hydrophobic. The start of protein synthesis in prokaryotic organisms requires a methionine residue. Met + Cys 0.91 g/day is the typical adult's daily nutritional need, and it is a limiting protein in most diets. If more than 4 percent of the protein is consumed, it turns poisonous.
This amino acid has an aromatic, nonpolar hydrophobic R-group, making it optimal for constituting the core of proteins. When present on the surface, it may facilitate substrate recognition or hydrophobic interactions with other molecules. Phenylketonuria is a debilitating condition that is believed to affect 1% of those with intellectual disabilities. The condition arises from the liver's inability to metabolize phenylalanine, necessitating that affected individuals refrain from consuming meals containing this amino acid. The scarcity of natural proteins without phenylalanine presents a significant technical problem in feeding individuals with phenylketonuria. Average individuals require 0.98 g/day of (Phe + Tyr).
This amino acid is required for humans because to its indole, nonpolar hydrophobic R-group, with an average adult need of 0.25 g per day. Plant proteins are likely deficient in tryptophan owing to the redirection of the indole group towards auxin production. Pellagra is now a rare condition that may arise in individuals whose diets consist of vegetable proteins deficient in tryptophan. Symptoms include cutaneous eruptions triggered by sunshine, diarrhea, profound neurological depression, and partial paralysis. The use of tryptophan or nicotinic acid mitigates these effects.
The second-simplest amino acid is this Alanine, which functions admirably in both hydrophobic and hydrophilic conditions due to its methyl side chain. Proteins that fold tightly, like keratin and collagen, have significant quantities of this amino acid, similar to glycine.
Despite its unusual structure, proline is not an essential amino acid. As a result of the nitrogen group forming a five-membered ring with the alpha carbon, proline possesses a secondary amine rather than the primary amine seen in other amino acids. Because of its unusual conformation, L-proline is endowed with crucial characteristics that impact the three-dimensional protein structure. The common precursor pyrroline-5-carboxylate (P5C) is used to generate proline from glutamine and ornithine. By introducing sharp twists into the polypeptide chain, proline causes a drastic shift in the protein's shape. As a result, proline serves as a signal for sections of protein structures that are functionally significant. The fact that proline motifs impose conformational limits on the polypeptide chain indicates that this amino acid is vital for key structural and biological activities. When it comes to metabolism, nutrition, cell recognition, and intracellular signaling, proline-rich proteins and peptides are among the most physiologically important.
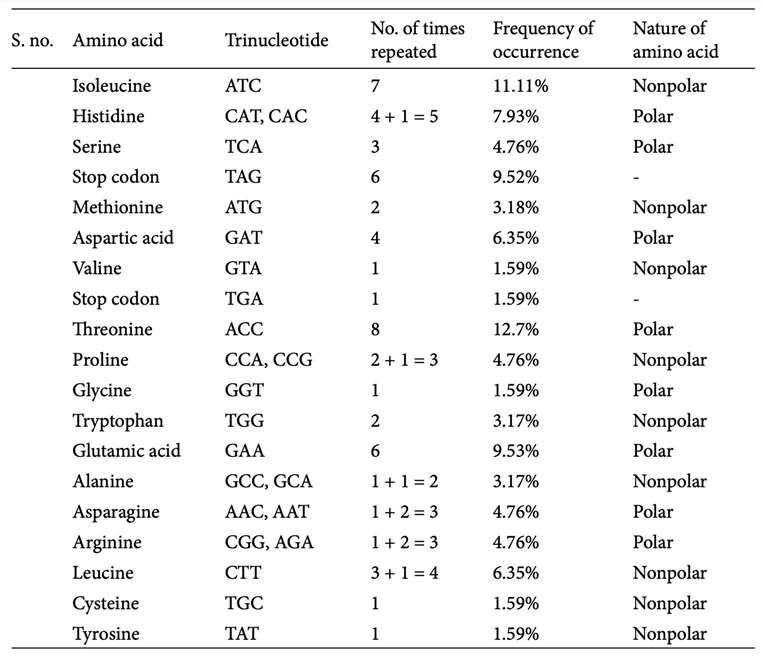 List of amino acids and their occurrence frequency. (Kaur R., et al., 2015)
List of amino acids and their occurrence frequency. (Kaur R., et al., 2015)
"Grandma Always Visits London In May For Waffles and Pancakes." The capital letter of each of these words corresponds to a nonpolar amino acid: G-Glycine (Gly, G); A-Alanine (Ala, A); V-Valine (Val, V); L-Leucine (Leu, L); I-Isoleucine (Ile, I); M-Methionine (Met, M); F-Phenylalanine (Phe, F); W-Tryptophan (Trp, W); P-Proline (Pro, P).
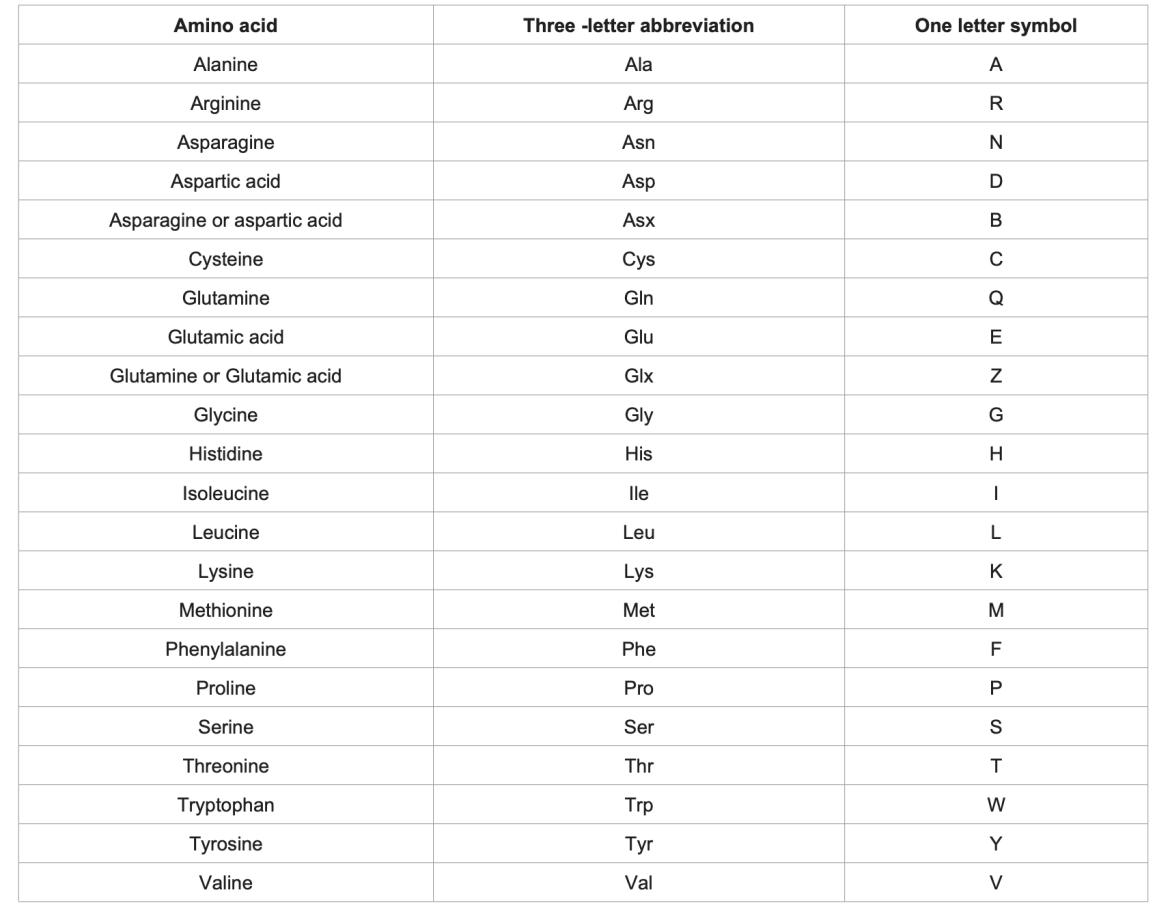 Three-letter and one letter abbreviations. (Khan R H., et al., 2017)
Three-letter and one letter abbreviations. (Khan R H., et al., 2017)
Hydrocarbons (alkyl or aromatic groups) or other nonpolar functional groups make up the majority of the side chains of nonpolar amino acids. The presence of nonpolar amino acids in proteins helps to sustain their structure by creating hydrophobic cores that evade water. Nonpolar amino acids interact with the hydrophobic lipid bilayer in proteins that form membranes. The side chains of nonpolar amino acids are electrically neutral at normal pH (~7.4) because they do not have a charge. The hydrophobic effect is caused by nonpolar amino acids. In order to reduce their exposure to water, hydrophobic residues cluster together in an aqueous environment. Proteins can't fold correctly or build functional three-dimensional structures without this clustering.
The majority of the amino acids in soluble elastin, also called tropoelastin, are nonpolar and uncharged. The molecular weight of the polypeptide chain ranges from 65 to 72 kDa, and it comprises 850 amino acids as expected. varying species have relatively varying real sizes. Remaining amino acid residues account for 86% of the total, with glycine making up 33%, alanine 18%, proline 13%, valine 17%, and leucine 5%. The relevance of the cotranslational alteration that occurs when 1-2% of proline residues are converted to hydroxyproline by prolyl hydroxylase remains unknown. Since there are around 36–38 lysine residues in a molecule, the monomer has a basic charge and an isoelectric point greater than pH 10. A chain reaction involving aldol condensations and Schiff bases forms the desmosine cross-links after lysyl oxidase deaminates the epsilon amino group of the majority of lysine residues in the protein. The protein is now permanently insoluble, and peptide bond cleavage is necessary for the extraction of soluble fragments.
A low net charge and mostly nonpolar amino acid composition characterize hydrophobic cell penetrating peptides (CPPs). A number of hydrophobic CPPs, such as α1-antitrypsin and fibroblast growth factor 12, originate from naturally occurring proteins. But there aren't many CPPs in this class, and synthetic peptide libraries are where most of the new hydrophobic CPPs come from. Chemically modified peptides, including stapled, prenylated, and pepducin peptides, are also included in this class. The use of these synthetic alterations to improve peptide absorption opens up promising avenues for the creation of cell-penetrating drugs derived from a diverse range of natural substances.
Although amino acids (AAs) primarily serve as building blocks for proteins, they may also play a role in nutrition metabolism as bioactive compounds. One group of amino acids that play a significant role in nutrition are the branched chain amino acids (BCAAs), which consist of leucine, isoleucine, and valine. These signals mediate various processes, including protein synthesis, glucose homeostasis, anti-obesity efforts, and nutrient-sensitive signaling pathways like phosphoinositide 3-kinase-protein kinase B (PI3K-AKT) and mammalian target of rapamycin (mTOR). Previous research has shown that glutamate transamination to alanine is one mechanism by which an increase in the catabolic flow of BCAAs could lead to glucose intolerance and enhanced gluconeogenesis. Obese people also have higher circulating BCAA levels, which are associated with poor metabolic health and an increased risk of insulin resistance (IR) and type 2 diabetic mellitus (T2DM). The researchers led by Ruiz-Canela found a direct correlation between high levels of BCAAs in the blood at the start of the study and an increased risk of cardiovascular diseases (CVDs). They also found that interventions based on the Mediterranean diet could reverse these harmful associations by changing pathophysiological processes and providing cardioprotective benefits. Obesity, insulin resistance, type 2 diabetes, and cardiovascular disease outcomes may be predicted using BCAAs as biomarkers. A growing number of targeted treatments aim to treat advanced cancers by blocking dysregulated signaling networks, such as the PI3K-AKT-mTOR signal pathway. According to previous research, AAs may stimulate Akt signaling via class I PI3K. This signaling route also activates mTORC2, which provides new information on the significance of this pathway in cell growth, proliferation, and survival. Beyond their obvious nutritional value, BCAAs have been shown in a small number of studies to have critical regulatory functions in a wide variety of activities, including the development of illness.
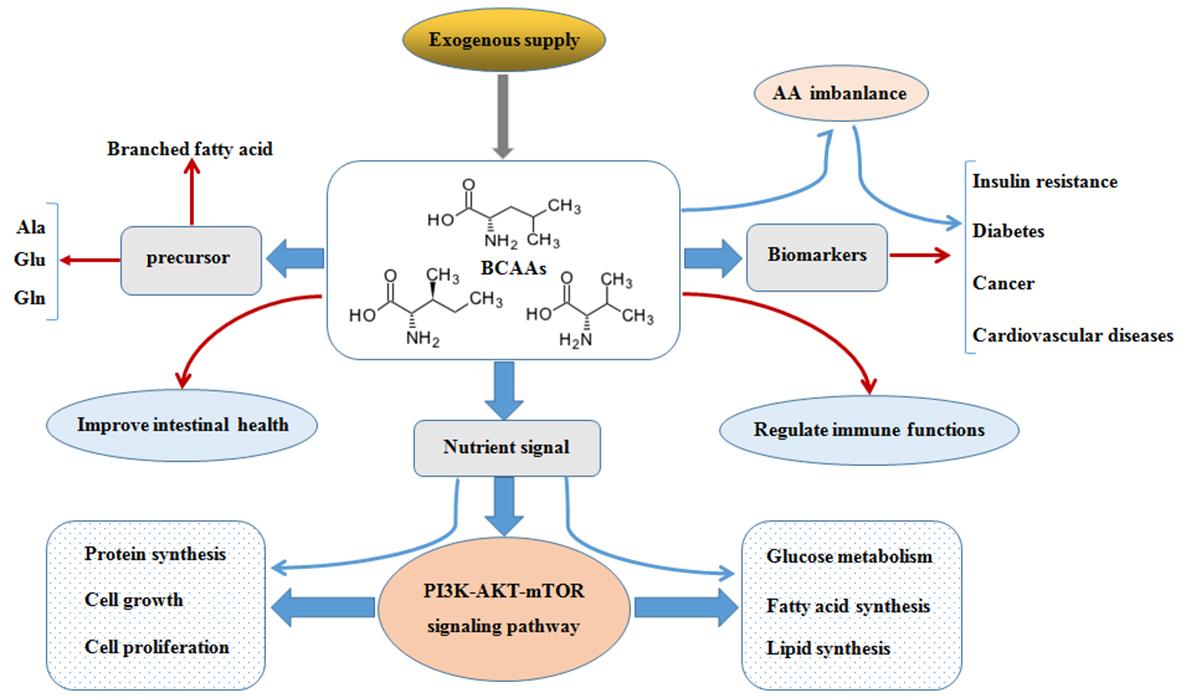 The functions of BCAAs. (Nie C., et al., 2018)
The functions of BCAAs. (Nie C., et al., 2018)
References