Among the variety of post-translational modifications (PTMs), the disulfide bond has gained considerable momentum in biological chemistry as it occurs instantaneously through oxidative folding in peptides, proteins, hormones, enzymes, growth factors, toxins, and immunoglobulins. A group of different disulfide-rich cyclic peptides has become a new focus of study in drug discovery and development field in recent years. These cyclic peptides provide an ideal scaffold for potential therapeutics.
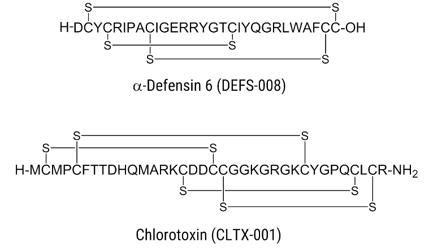 Fig.1 The structure of two disulfide-rich peptides: α-Defensin 6 and Chlorotoxin
Fig.1 The structure of two disulfide-rich peptides: α-Defensin 6 and Chlorotoxin
The artificial formation of disulfide bridges requires the proper management of cysteine residues, including first protecting and then later removing side groups and properly pairing the cysteine residues. Cysteine is the prevailing site for covalent PTM in peptides and proteins.
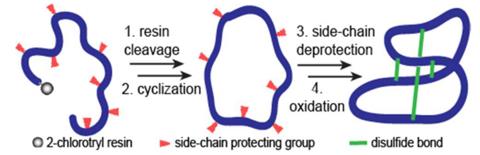 Fig. 2 The formation of disulfide bond (Olivier Cheneval et al, 2014)
Fig. 2 The formation of disulfide bond (Olivier Cheneval et al, 2014)
Creative Peptides provides multiple methods for synthesizing disulfide-rich cyclic peptides:
Creative Peptides specialized in the custom synthesis of disulfide-rich cyclic peptides, providing a confidential and efficient service at competitive prices. Every step of peptide synthesis is subject to Creative Peptides' stringent quality control. Typical delivery specifications include:
Disulfide-rich cyclic peptides are peptides that contain one or more disulfide bonds, which stabilize their structure and enhance their biological activity. These peptides are valuable in research and drug development due to their increased stability, target selectivity, and potential for therapeutic applications, particularly in enzyme inhibition and molecular recognition.
Disulfide-rich cyclic peptides are synthesized using methods like classical peptide synthesis (solution or solid phase), followed by oxidation to form disulfide bonds. Additionally, Ugi multicomponent reactions and chemical ligation can be employed to introduce disulfide bonds, with specific management of cysteine residues to ensure proper bond formation.
Disulfide bonds enhance the potency, rigidity, target selectivity, and stability of cyclic peptides. These bonds stabilize the secondary structure of peptides, making them more resistant to degradation. They also serve as a common structural motif in many biologically active compounds, improving their therapeutic potential.
Yes, Creative Peptides specializes in synthesizing disulfide-rich cyclic peptides with up to four disulfide bonds. This allows for the creation of peptides with enhanced stability and functionality, ideal for structural studies and various research applications.
Disulfide-rich cyclic peptides are widely used in structural studies, enzyme function analysis, and the investigation of biological functions. They are also valuable in studying protein interactions and molecular recognition, with potential uses in diagnostics and as tools for drug discovery.
At Creative Peptides, each step of the synthesis process undergoes stringent quality control. Our typical delivery specifications include HPLC chromatograms, mass spectrometry analysis, synthesis reports, and certificates of analysis, ensuring that every peptide meets the required purity and functionality.
We use several methods for synthesizing disulfide-rich cyclic peptides, including solid-phase peptide synthesis (SPPS), solution-phase synthesis, native chemical ligation, and Ugi multicomponent reactions (U-MCR). These methods allow for both intermolecular (dimer formation) and intramolecular (cyclic peptide formation) disulfide bond incorporation.

References