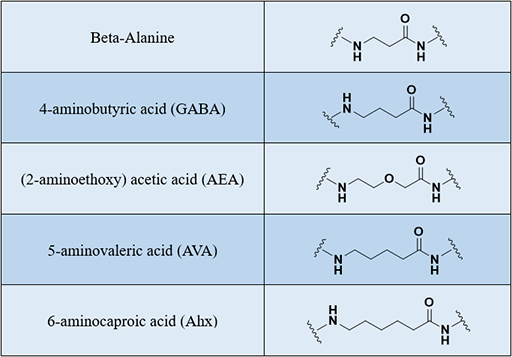
Linkers, we can also call spacers, which can be used to link two molecules of interest to us. They are always flexible or stretched molecule. Depending on the field of design and application, we can construct plugins in peptides and different molecules to construct molecular structures that can be used for research. Generally, we can insert a linker between the peptide and the following molecules:
Different lengths of linkers can bind to any site on the peptide, usually via an amide bond. One common hydrophobic spacer is aminocaproic acid (Ahx), a common hydrophilic spacer is polyethylene glycol (PEG), and there are many other options. In recent years, PEGylation has been widely used method for improving the stability and bioavailability of peptides in vivo. If you need, please visit Peptide PEGylation.
Creative Peptides can provide linkers as shown below, including but not limited to the following examples:
Inserting a linker between peptide and other molecules can help:
Linkers play a crucial role in peptide conjugation by providing the necessary flexibility and distance between the peptide and the attached molecule. This allows for proper interaction between the peptide and its target while preventing steric hindrance. Linkers ensure that both components retain their functionality and stability during experiments or industrial applications.
PEG linkers are widely used in peptide synthesis because they are hydrophilic, which improves the solubility and stability of peptides in aqueous environments. PEGylation reduces aggregation, enhances bioavailability, and increases the half-life of peptides in vivo. This makes PEG linkers ideal for enhancing peptide stability during biological studies and applications.
Hydrophobic linkers, like aminocaproic acid (Ahx), are typically used when a more rigid structure is required for peptide conjugation. They help improve the binding efficiency and target specificity. Hydrophilic linkers, like PEG, are used to enhance solubility, reduce aggregation, and improve the overall stability of peptides in aqueous environments, especially in biological applications.
Yes, linkers can be used to connect peptides to larger biomolecules like carrier proteins (e.g., KLH, BSA) or antibodies. By incorporating a linker, the peptide can maintain its structural integrity and functional activity while being attached to a larger biomolecule, which is essential for applications like immunization, detection, and protein interaction studies.
The spacer length directly influences the flexibility and distance between the peptide and its conjugated molecule. Short spacers may limit flexibility, while longer spacers can provide more freedom for the peptide to interact with its target. The optimal spacer length depends on the specific requirements of the experiment or application, such as improving binding affinity or maintaining peptide bioactivity.
Using linkers in peptide-based drug development offers several advantages, including improved specificity, stability, and controlled release. Linkers ensure that the peptide remains functional when conjugated to other molecules, which is important for applications like targeted drug delivery, diagnostics, and proteomic studies.

References