Spin labeling refers to a chemical technique that integrates molecules containing unpaired electrons into another research framework. For the study of biomacromolecule structure, kinetics and conformational changes, this technique provides a relatively non-invasive technique. The combination of site-directed spin labeling (SDSL) and electron paramagnetic resonance spectroscopy (EPR) is a mature method and has been widely used in recent years. There are many applications in protein and peptide science.
In general, a paramagnetic substance can be used as a paramagnetic detection probe as long as it can be attached to a biological macromolecule such as a protein. Diamagnetic molecules can be studied by attaching a molecule containing an unpaired electron, which known as spin labelling. In the EPR study of peptide, the preferred paramagnetic probe is referred to as a peptide spin label. The peptide spin labels commonly found in biomacromolecules can be broadly classified into two types: metals having paramagnetism and organic radicals containing unpaired electrons.
Most EPR methods rely on the use of spin labeling reagents, typically unpaired electrons are typically provided by nitroxide radicals and are shielded to maintain stability. EPR technology allows small magnetic coupling to be measured providing information on single-label probes or dipole coupling between multiple labels.
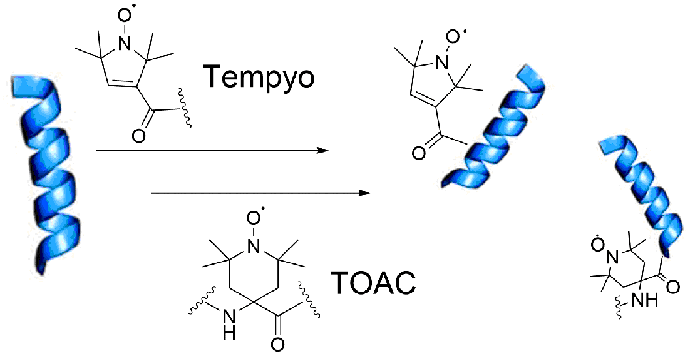
In general, nitroxides such as TEMPYO (2,2,6,6-tetramethyl-1-piperidinyloxy) derivatives are used more because they provide high unpaired electron stability and special ESR sensitivity. While TOAC can be used as an analog of any amino acid building block and placed anywhere in the peptide chain
We also offer the following spin labeling reagents:
Creative Peptides provides our customers with high-quality spin labeling services and combine spin labels to specific sites according to your needs. At the same time, all processes meet the highest quality assurance to ensure high quality spin-labeled peptides.
Peptide spin labeling is a technique that incorporates molecules containing unpaired electrons into peptides, enabling the study of their structure, kinetics, and conformational changes. This non-invasive technique is commonly used with electron paramagnetic resonance (EPR) spectroscopy to analyze peptides and proteins.
In spin labeling, a paramagnetic probe, often a nitroxide radical, is attached to a specific site on the peptide. This probe contains unpaired electrons, allowing the use of EPR spectroscopy to detect magnetic interactions and obtain structural and dynamic information about the peptide.
The most common spin labels used for peptides include nitroxide radicals, such as TEMPYO (2,2,6,6-tetramethyl-1-piperidinyloxy) and TOAC (2,2,2-trifluoroethylthio-4-aminomethylphenyl), which are known for their stability and sensitivity in EPR analysis.
Peptide spin labeling is widely used in structural biology, protein chemistry, and biophysics. It helps study protein folding, conformational changes, molecular dynamics, and interactions at the atomic level. This technique provides insights into protein function and structure that are difficult to achieve with traditional methods.
Creative Peptides offers a variety of spin labeling reagents, including 4-Carboxy-TEMPO, 16-DOXYL-stearic acid, 4-Hydroxy-TEMPO, and 4-(2-Iodoacetamido)-TEMPO. These reagents provide different functional groups to suit various labeling requirements for EPR spectroscopy.
Yes, Creative Peptides provides custom peptide spin labeling services. We can incorporate spin labels at specific sites on your peptide, ensuring that the labeling meets your research needs, whether for single-label probes or multiple-label dipole coupling studies.
Creative Peptides adheres to strict quality control protocols for spin-labeled peptides. Each peptide is synthesized and labeled with high precision to ensure the integrity and stability of the spin labels. We also provide HPLC and mass spectrometry analyses for quality assurance.

References