2024-07-09
Introduction
Aviptadil acetate, the nonproprietary or generic name for a vasoactive intestinal peptide (VIP), is a synthetic 28-amino-acid VIP (Fig.1). In pulmonary arterial hypertension (PAH), elevated pulmonary vascular resistance and pulmonary arterial pressure are resulting from the progressive narrowing of the pulmonary arterial lumens. Although an array of anti-PAH drugs are currently available, PAH therapy still suffers from a number of limitations. Thus a series of small molecular weight drugs and peptides have been evaluated for their anti-PAH effects. Inhalation of aviptadil has been suggested as a novel approach for the treatment of idiopathic PAH. Idiopathic PAH patients are deficient in VIP that triggers a compensatory up regulation of receptor expression in the pulmonary vasculature to counter-regulate VIP deficiency.
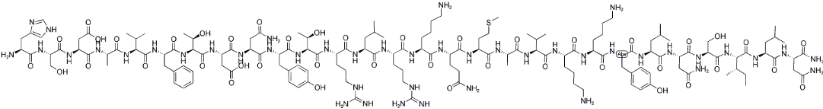
Fig. 1 The general structure of aviptadil acetate
Functional Study of Aviptadil Acetate on PAH
There were many experimental studies about the function of Aviptadil Acetate on PAH. One trial described the acute effects on haemodynamics and blood gases, as well as the tolerability, of a single 100-mg inhaled dose of aviptadil in a mixed group of patients with moderate to severe PAH. Aviptadil was well tolerated, acted as a weak pulmonary selective vasodilator and alleviated right heart strain. These effects were comparable in the PAH and the non-PAH group. Additionally, aviptadil tended to improve oxygenation in patients with chronic lung disease, which suggested further studies were necessary to evaluate the role of aviptadil as a new therapeutic option in the field of PAH. Another trial concluded that patients with idiopathic PAH lack VIP and have a compensatory upregulation of receptor expression in the pulmonary vasculature, as an attempt to counter-regulate VIP deficiency. Subsequently, eight patients with idiopathic PAH were treated and it also observed an acute and a chronic effect of aerosolised aviptadil. In an acute challenge with a single 100 μg dose of aviptadil during right heart catheterization, a P-pa reduction of 10 mmHg and an increase of cardiac output of 0.8 L/min were observed. Chronic treatment with aviptadil comprised four inhalations per day, equaling a daily dose of 200 μg. After a period of 12 weeks a significant improvement of the 6 min walk test was noted. Although the present authors administered the same dose of aviptadil, comparable strong effects with regard to pulmonary vasodilation were not seen. However, in the PAH group the increase in cardiac output (~0.6 L/min ) was comparable. The modest vasorelaxant effect of a single 100 μg aviptadil inhalation may not reflect the full therapeutic potential of this drug. Additional study provided experimental evidence that VIP is involved in the proliferation process of pulmonary vascular smooth muscle. They were able to demonstrate that VIP deficiency leads to moderately severe PAH in male mice lacking the VIP gene. This observation may justify the speculation that chronic treatment with aviptadil positively influences the remodeling process in the pulmonary vasculature in the context of PAH.
The Wide Acceptance
Biogen Idec, the drug producer in the United States, and Mondo Biotech AG, the biotechnology company in Switzerland, have signed a sole transfer and cooperation agreement, which coveres the development and production of Aviptadil for the treatment of pulmonary hypertension. Aviptadil has been used for pulmonary arterial hypertension in the United States and Europe as a rare drug. Results in phase I trials showed that the patients’ athletic ability had improved significantly since they undergone the treatment of Aviptadil for three to six months.
References:
Petkov, V., Mosgoeller, W., Ziesche, R., Raderer, M., and Stiebellehner, L. (2003). 'Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension’, Journal of Clinical Investigation, 111(9): 1339-1346.
Said, S.I., Hamidi. S.A., Dickman, K.G.,Szema, A. M., Lyubsky, S. (2007). 'Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene’, Circulation, 115(10): 1260-1268.
Leuchte, H.H., Baezner, C., Baumgartner, R. A., Bevec, D. Bacher, G. (2008). 'Inhalation of vasoactive intestinal peptide in pulmonary hypertension’, European Respiratory Journa, 32(5): 1289-1294.