Peptide bonds, also known as amide bonds, are covalent chemical bonds that link amino acids together in proteins. They are formed through a specific type of reaction called a condensation reaction or dehydration synthesis. In this reaction, the carboxyl group (-COOH) of one amino acid reacts with the amino group (-NH2) of another amino acid, resulting in the formation of a peptide bond and the release of a molecule of water (H2O).
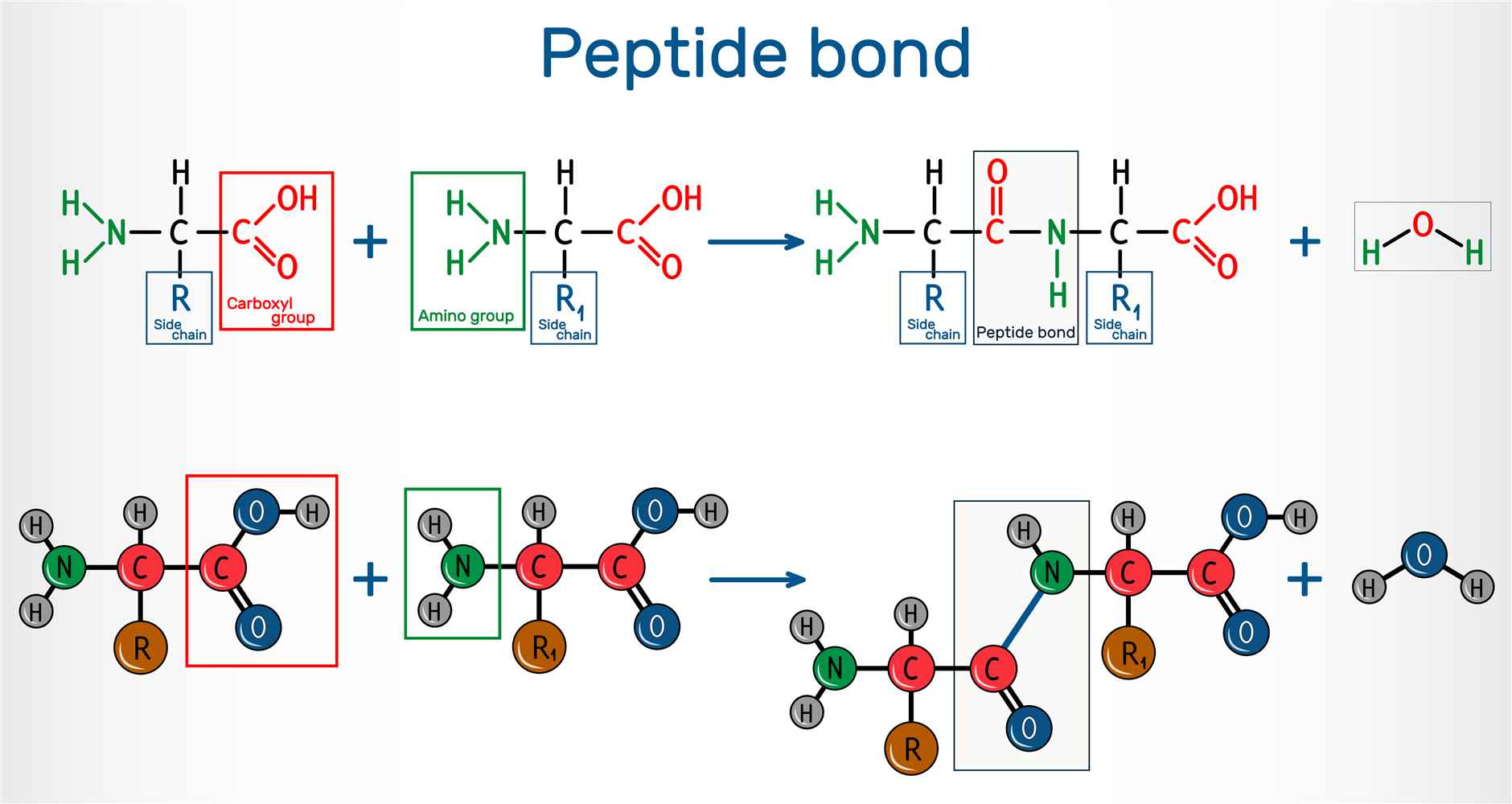 Fig. 1 Synthesis of recombinant peptide
Fig. 1 Synthesis of recombinant peptide
Peptide bond formation, also known as peptide bond synthesis or peptide bond formation, is a vital process in the biosynthesis of proteins. It occurs during protein translation, specifically in the elongation phase, and involves the formation of a covalent bond between two amino acids. The formation of a peptide bond involves the nucleophilic attack of the amino group on the carbonyl carbon of the carboxyl group. This process leads to the formation of a new bond between the carbon and the nitrogen atoms, while the oxygen atom becomes a part of a carbonyl group in the peptide bond. The resulting structure is a peptide linkage, which connects the α-carbon of one amino acid to the nitrogen atom of the next amino acid in the protein chain.
Peptide bonds exhibit unique conformational properties that significantly influence the structure and function of proteins. These properties include planarity, resonance, and isomerization. Understanding these conformational characteristics is crucial for comprehending protein folding, stability, and the ability to adopt specific three-dimensional structures. The key conformational properties of peptide bonds are as follows:
Planarity: Peptide bonds are characterized by their planarity. The peptide bond exhibits a rigid, nearly planar structure due to the partial double bond character resulting from resonance stabilization. This planarity arises from the alignment of the p orbitals of the carbonyl oxygen and the nitrogen atom involved in the bond formation. The planar arrangement of the peptide bond influences the folding and geometry of the protein backbone.
Resonance: Peptide bonds exhibit resonance, which is a result of electron delocalization within the bond. The electron density is shared between the carbonyl oxygen and the nitrogen atom, creating a resonance structure with double bond character between the carbon and nitrogen atoms. This resonance contributes to the stability of the peptide bond and reduces the ability of the bond to rotate freely, resulting in limited rotation around the peptide bond.
Trans and Cis Isomerization: Peptide bonds predominantly adopt the trans configuration, where the amino and carbonyl groups are positioned on opposite sides of the peptide bond. This trans conformation is more energetically favorable due to steric hindrance between the amino hydrogen and the carbonyl oxygen in the cis conformation. However, in certain cases, peptide bonds can adopt the cis configuration, leading to a kink or bend in the protein backbone. Cis isomerization is typically facilitated by specific proline residues, which have a unique conformational preference due to their cyclic structure.
Ramachandran Plot: The conformational flexibility of the peptide bond is further illustrated by the Ramachandran plot, which displays the allowed regions of backbone dihedral angles (phi and psi) in a protein. The plot reveals the preferred regions of rotation for the peptide bond, highlighting the energetically favorable conformations. The Ramachandran plot provides insights into protein folding and helps predict the secondary structure elements, such as alpha helices and beta sheets, based on the dihedral angles of the peptide bonds.
Peptide bonds are relatively stable under physiological conditions due to their resonance and partial double bond character. The resonance structure resulting from electron delocalization within the peptide bond contributes to its stability. This stability is further enhanced by the planarity of the peptide bond, which restricts rotation and minimizes strain in the bond. The stability of peptide bonds allows proteins to maintain their structural integrity over extended periods.
Despite their stability, peptide bonds can undergo hydrolysis, which involves the cleavage of the bond by the addition of a water molecule.
 Fig. 1 Synthesis of recombinant peptide
Fig. 1 Synthesis of recombinant peptide
Peptide bonds provide the backbone of protein structures. The linear arrangement of amino acids connected by peptide bonds determines the primary structure of proteins, which subsequently influences the folding, stability, and three-dimensional conformation of proteins. The specific sequence of amino acids dictated by peptide bonds determines the overall protein structure, allowing proteins to adopt unique shapes and carry out specific functions.
Peptide bonds play a fundamental role in protein folding. The sequence of amino acids connected by peptide bonds determines the folding pathway of proteins, allowing them to achieve their native, functional conformation. The hydrophobic effect, hydrogen bonding, and other non-covalent interactions influenced by peptide bonds contribute to the folding process, enabling proteins to attain their stable three-dimensional structures.
Peptide bonds are essential for the catalytic function of enzymes. Many enzymes contain an active site where peptide bonds are involved in binding and modifying substrates. The active site of an enzyme may cleave peptide bonds, create new peptide bonds, or facilitate other chemical reactions. Enzymes catalyze these reactions by utilizing the precise arrangement of amino acids connected by peptide bonds within their active sites.
Peptide bonds play a critical role in mediating protein-protein interactions. Proteins often interact with other proteins to carry out specific biological functions. Peptide bonds within interacting proteins contribute to the formation of interfaces and binding sites that allow proteins to recognize, bind to, and interact with other molecules. These interactions are crucial for various cellular processes, including signal transduction, gene regulation, and cellular transport.
Peptide bonds are involved in protein degradation and turnover. Proteins have a finite lifespan and undergo degradation to maintain cellular homeostasis. Proteases, enzymes that specifically cleave peptide bonds, target specific sequences or structural motifs within proteins for degradation. Peptide bond hydrolysis carried out by proteases breaks down proteins into smaller peptides and amino acids, allowing recycling of the building blocks for protein synthesis.
Peptide bonds are crucial for the function of peptide hormones and signaling molecules. Peptide hormones, such as insulin and glucagon, consist of short peptide chains connected by peptide bonds. These hormones play vital roles in regulating physiological processes such as metabolism, growth, and development. Peptide bonds within these hormones are essential for their stability, structure, and binding to specific receptors.
Peptide bonds contribute to the generation of antibody diversity. Antibodies, produced by the immune system, recognize and neutralize specific foreign molecules (antigens). The variable region of an antibody, responsible for antigen recognition, is generated through a process called V(D)J recombination. During this process, different gene segments encoding antibody variable regions are rearranged, resulting in the formation of peptide bonds that determine the antigen-binding specificity of antibodies.