Side chain (SC) cyclization is a method utilizing functional groups such as amines, carboxylic acids or thiols, or the amino or carboxyl termini of the peptide to form the cyclization through amide or disulfide bonds.
Both the cyclization types have their respective advantages: The backbone cyclization (BC) peptide is more active and stable, probably due to the benefit of N-alkylation. But SC cyclic peptides are easier to synthesize. To a certain extent, the BC scaffold can be replaced by the SC one. It is essential for drug design to compare the properties of SC versus BC peptides, since cyclization is one of the most frequently used methods for peptido- and proteinomimetics. The synthetic disadvantages of BC make this method sometimes difficult to approach, which needs the improvement such as the coupling methods and building blocks accessibility. For SC cyclization, the minimum length of the peptide is 4 amino acid units, but the maximum length is not limited.
Side chain cyclization is the classical ones that are limited to cyclization through the side chains and/or the amino or carboxyl terminal groups. Conformationally restricted peptides which contain medium- and long-range cyclization have been mainly prepared following the same modes of cyclization of homodetic and heterodetic natural peptides. Common methods of side chain cyclization are:
Creative Peptides specialized in the process development and the manufacturing of bioactive peptides. We are dedicated to offering custom peptide synthesis, process development, GMP manufacturing as well as catalog products for customers in industry and research area. We have more than 20 years of experience in peptide research, and we are dedicated to provide high-quality GMP peptides to researchers and pharmaceutical companies all over the world.
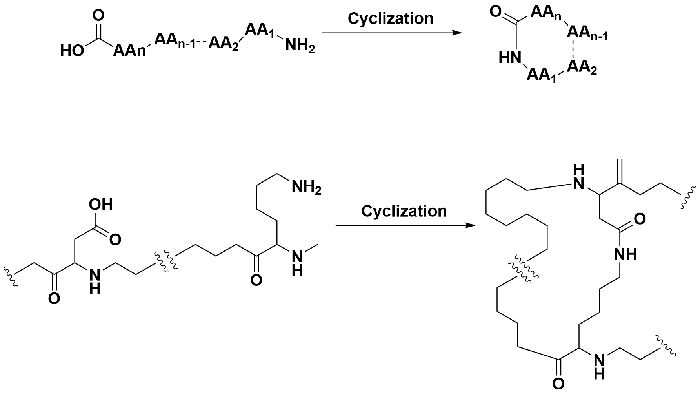
Side chain cyclization is a method where functional groups like amines, carboxylic acids, or thiols on the peptide's side chains or termini form a cyclization via amide or disulfide bonds, resulting in a cyclic peptide with enhanced stability and bioactivity.
While both cyclization methods enhance peptide stability and activity, side chain cyclization is easier to synthesize and involves functional groups on the peptide's side chains, whereas backbone cyclization uses the peptide's main chain for cyclization, often resulting in greater stability and activity.
Side chain cyclization is more straightforward to synthesize compared to backbone cyclization, and it allows for the creation of cyclic peptides with improved stability, specificity, and function. It can be used to enhance the pharmacological properties of peptides while maintaining ease of production.
Common methods include Cys-Cys cyclization, amide bond cyclization, and the formation of lactam rings or disulfide bonds. These methods can be tailored based on the peptide sequence and the desired cyclization outcome.
The minimum length for effective side chain cyclization is typically 4 amino acid units, but there is no strict maximum length, allowing for flexible design depending on the peptide's function and application.
Side chain cyclization can involve amines, carboxylic acids, thiols, or the amino and carboxyl termini of peptides, depending on the desired type of bond (amide, disulfide, etc.) and the final structure of the cyclic peptide.
Side chain cyclization is an essential tool in drug design, particularly for peptide and protein mimetics, as it enhances the peptide's bioactivity, stability, and specificity. It can be used to create peptides that are more resistant to enzymatic degradation, improving their therapeutic potential.

References