Cell penetrating peptides are a class of short peptides with a length of 5~30 amino acids, which can carry polypeptides, nucleic acids, small molecules of drugs and virus particles through cell membranes into cells. People use it as a carrier to transport objects into cells. Past research has proven effective in treating mouse models of cancer and inflammatory diseases with cells that carry proteins and peptides through peptides. Based on animal studies, it is thought that it will be possible for cells to penetrate peptides carrying DNA or SiRNA to treat disease.
Cell-penetrating peptides can also improve the efficiency of viral transfection. Therefore, for this can help treat the disease, but also may facilitate the spread of the virus, the double-edged sword, people need to increase research efforts to avoid harm. In addition,cell-penetrating peptides can carry fluorescent or radioactive reagents for imaging applications. In conclusion, cell-penetrating peptides carrying therapeutic genes or drug molecules into cells could have a very broad clinical application prospect.
The molecular design of conventional cell-penetrating peptides cannot be separated by three key parameters: guanidinium content (or cationic amino acid content), hydrophobicity and amphipathicity. Cell penetrating peptides rich in arginine (arginine with a guanidinium side chain, pKa of approximately 12 and protonated guanidinium cation at physiological pH) have been intensively studied in the field of primary structure-potency.
Membrane models, membrane extracts and in vivo studies have played a key role in investigating the amino acid sequence and the contribution of individual amino acid residues (e.g. positive charge) of cell-penetrating peptides, these studies have guided the molecular design of cell-penetrating peptides. Despite these theoretical guidelines, it is still very difficult to predict the membrane penetration of a peptide from its sequence alone.
Except for the sequence. A variety of chemical and physico-chemical properties, such as charge, chirality, aromaticity and hydrophobicity, and their interactions are often important driving forces for the internalisation of cell-penetrating peptides. It can be said that the peptide sequence is the basis on which the intermolecular interactions determine the efficacy of the cell-penetrating peptide. To design efficient cell-penetrating peptides, numerous parameters including charge, guanidine groups, chirality, hydrophobicity and aromaticity, and their interactions, need to be further investigated in depth.
Peptide sequences rich in side chain cationic amino acids are important in the field of cellular penetration of peptides. The discovery of Tat peptide was a landmark event in the field of cell-penetrating peptides. Tat (Transactivating transcriptional activator) peptide is a peptide fragment of residues 48 to 60 of the original transcriptional activator of human immunodeficiency virus (HIV-1). Tat's modified gene delivery system shows enhanced transport across multiple biofilms, such as cell membranes, endosomal membranes, and nuclear membranes.
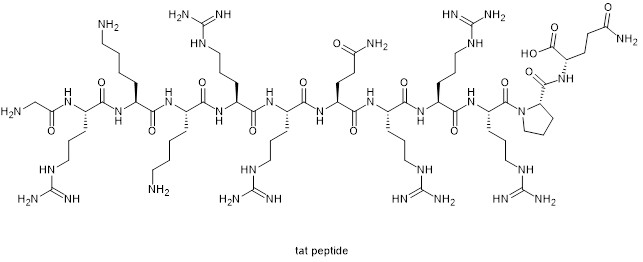 Chemical structure of Tat peptide
Chemical structure of Tat peptide
Tat peptide contains six arginine and two lysine residues, all of which belong to basic amino acids. The side chains are guanidine and amino respectively, and all of them are positively charged due to protonation under physiological pH conditions. Given the significant guiding importance of Tat, it is not surprising that the simplest cell-penetrating peptide mimic was designed as an oligoarginine.
A correlation between the internalisation effect of cell-penetrating peptides and cationic residues has been demonstrated. Inspired by Tat (GRKKRRQRRRPQ) and osmolytes (RQIKIWFQNRRMKWKK), the molecular design of cell-penetrating peptides has focused on small cationic peptides (carrying around 5 positive charges) such as polyarginine and polylysine peptides.
In addition to the cationic part, the lipophilic part is also important for cellular uptake. In this regard, researchers have proposed a mechanism for the internalization of guanidine-rich cell penetrating peptides by the formation of hydrophobic inverse (negative) ions near the main chain. The counterion effect is first appears as the hydrogen bond interaction between arginine residues and membrane components, forming a complex that can cross the membrane. In contrast, no such strong counterion binding was observed in the lysine-rich peptide, a finding which in turn demonstrates the relevance of the guanidinium counterion for promoting the internalisation of cell-penetrating peptides. This property of cell-penetrating peptides to bind hydrophobic counterions is referred to as the self-activating property of these peptides. Therefore, while focusing on the positive peptides charge, it is important not to neglect the hydrophobicity studies of cell-penetrating peptides and the contribution of single aliphatic and aromatic functional groups.
Hydrophobicity can be achieved by the addition of aliphatic or aromatic structures. Some studies of cell-penetrating peptide mimics have confirmed that aromatic activation is superior to aliphatic. Lipidization occurs by attaching hydrocarbon chains of varying lengths (alkyls) to the N-terminus or other suitable functional groups of peptides known to penetrate cells. This alkylation modification improves the internalisation of the cell-penetrating peptide by enhancing hydrophobic interactions with the membrane. In addition to the integration of aliphatic chains, the hydrophobicity of peptide molecules can also be improved by the introduction of hydrophobic amino acids (e.g. leucine, isoleucine, alanine, valine, phenylalanine). One cell has been reported to be three times more active in penetrating peptides after replacing the methyl group with the more hydrophobic butyl group.Correspondingly, in the same study, the internalization efficiency was improved when γ-dimethylsilaproline replaced proline Pro to construct a cell-penetrating peptide with a polyproline PPII helical structure. This result may be attributed to an overall increase in hydrophobicity of the peptide.
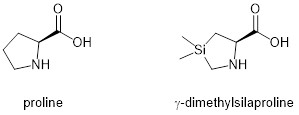 Chemical structure of proline and γ-dimethylsilaproline
Chemical structure of proline and γ-dimethylsilaproline
A hydrophobic viral peptide gH 625 (sequence: HGLASTLTRWAHYNALIRAF) with cell-penetrating properties has recently been reported. This cell-penetrating peptide was designed to transport liposomes, quantum dots, dendrimers, disordered proteins and SPIONS (Superparamagnetic iron oxide nanoparticles), which are derived from herpes simplex virus type I. The amphiphilic nature of the chemical structure is also evident.
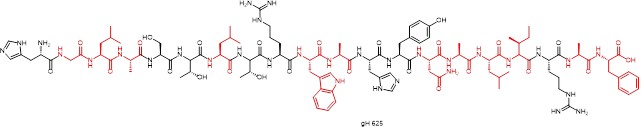 Structure of the hydrophobic cell-penetrating peptide Gh 625 (hydrophobic amino acid residues in red)
Structure of the hydrophobic cell-penetrating peptide Gh 625 (hydrophobic amino acid residues in red)
Peptide hydrophobicity can also be enhanced by the introduction of aromatic amino acid residues (tryptophan, phenylalanine and tyrosine). According to Wimley and White, aromatic residues have a dominant advantage of free energy during the insertion of the polypeptide into the double-layer interface of the cell membrane.Pyrene, halo-benzene and fullerenes have been shown to stimulate the activation of guanidinium-based cations, thereby allowing arginine-rich cell-penetrating peptides to cross the cell membrane.
The researchers then used a series of experiments to verify whether aromatic functional groups provided better internalisation efficiency than aliphatic functional groups, while maintaining the same hydrophobicity of the peptide. These studies found that aromatic functional groups facilitate the internalisation of cell-penetrating peptides more than aliphatic chains. Because aromatic peptides can interact π-π with aromatic amino acids on membrane proteins, they may help to facilitate or stabilise peptide-membrane interactions and aid internalisation.8 This concept has been extended to the effect of various π interactions (π-π, π-cation, π-anion and π-dipole) on the internalisation of peptide molecules.
In the molecular design of cell-permeable peptides, cationic and hydrophobic characteristics need to be considered together. The osmopeptide penetratin is an example of this. Osmin is obtained from a Drosophila homology domain protein. The non-electrostatic interaction of osmolyte with the non-polar part of the plasma membrane is important for internalisation (this also explains the basis for the design of the amphiphilic molecule, which contains non-polar amino acid residues such as leucine, tryptophan and phenylalanine in osmosis). The number of cationic residues, their spacing on the primary structure, their relative position on the secondary structure, and the integration of non-peptide elements containing, for example, hydrophobic lipid fractions, are crucial for the molecular design of cell-penetrating peptides.
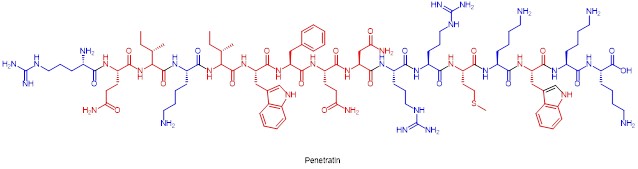 Chemical structure of osmolyte (amino acid residues with cations in blue, hydrophobic structure in red)
Chemical structure of osmolyte (amino acid residues with cations in blue, hydrophobic structure in red)
Another example is Pep-1, which consists of five tryptophan (a hydrophobic amino acid to enhance the hydrophobic interaction of the peptide with the cell membrane) and a positively charged lysine-rich fragment (KKKRKV, from the nuclear localization sequence on the viral SV-40 T-antigen), as well as a proline spacer to increase the flexibility of the peptide.
Similarly, the MPG peptide (GALFLGFLGAAGSTMGAWSQPKKKRKV) was designed to contain the same hydrophilic nucleolocalisation sequence, which was coupled to a hydrophobic sequence of the HIV-gp-41 viral fragment. By integrating cationic amino acid residues, hydrophobic fragments, and amphiphilic structures in different ways into the same peptide molecule, more efficient cell-penetrating peptides can be constructed and derived.
Advances have been made in the design of cell-penetrating peptides in response to challenges in the realisation of their function, such as internalisation efficiency, endosomal escape efficiency, cycle time, and specificity and selectivity (for cells, tissues, diseases). One example of this is the cysteine-rich (Cys) cell-penetrating peptide.
The molecular design was inspired by Crotamine, a toxin found in snake venom, which contains two nuclear localisation domains (crot(2-18) and crot(27-39)). By examining crot (27-39) (sequence: KMDCRWRWKCCKK), the researchers used systematic substitution and/or omission of amino acid residues, as well as in-depth conformational studies, to design a cysteine-rich decapeptide (CRWRWKCCKK) molecule (Figure 9). This potential cell-penetrating peptide has enhanced internalization efficiency.
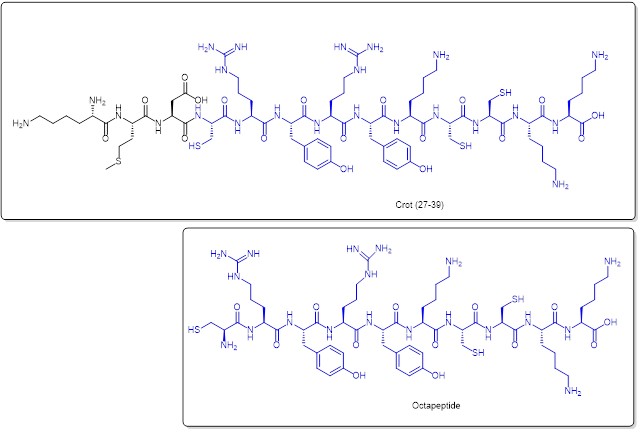 Comparison of the molecular structure of the Crotamine nuclear localisation fragment Crot (27-39) (above) with the cell-penetrating decapeptide (below) (preserved in blue).
Comparison of the molecular structure of the Crotamine nuclear localisation fragment Crot (27-39) (above) with the cell-penetrating decapeptide (below) (preserved in blue).
Cell-penetrating peptides have become a popular area of research for intracellular therapeutic agents. Because of the natural limitations of straight-chain cell-penetrating peptides, such as endosomal encapsulation, toxicity, poor cell specificity, poor stability and degradability, as well as imperfect cell penetration, modified cell-penetrating cyclic peptides have emerged. Compared to their straight-chain precursors, cell-penetrating cyclic peptides offer enhanced cell penetration and improved physical and chemical properties, as well as stability against hydrolytic degradation. Some cell-penetrating cyclic peptides can exhibit endosome-independent uptake, and nuclear targeting properties have been reported for some cell-penetrating cyclic peptides.
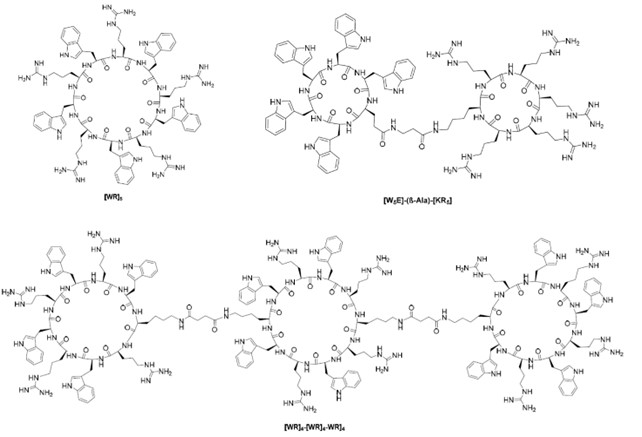 Chemical structures of monocyclic, bicyclic and tricyclic cell-penetrating cyclic peptides containing arginine and tryptophan residues.
Chemical structures of monocyclic, bicyclic and tricyclic cell-penetrating cyclic peptides containing arginine and tryptophan residues.
The two cell-penetrating cyclic peptide structural units [WR]4 and [WR]5 are characterised by the presence of alternating positively charged (arginine) and hydrophobic amino acids (tryptophan) in the sequence. Monocyclic cell-penetrating cyclic peptides containing tryptophan and arginine residues can also be coupled to potential therapeutic agents. For example, monocyclic peptides are coupled to doxorubicin, paclitaxel and camptothecin, where the doxorubicin-cyclic peptide adduct demonstrates the effect of internalisation.
In addition to this, several monocyclic peptides containing cysteine and arginine residues significantly enhanced the uptake of the impermeable cellular phosphopeptide (F')-Gly-(pTyr)-Glu-Glu-Ile (F'-GpYEEI). Cyclic decapeptides containing tryptophan and histidine effectively increased the intracellular delivery of the cell impermeable phosphopeptide with the anti-HIV drug emtricitabine (emtricitabine). The [WR]4-[WR]4-[WR]4 tricyclic peptide containing alternating arginine and tryptophan residues increased the cellular uptake of F'-GpYEEI and fluorescently labelled anti-HIV drugs (lamivudine (3TC), emtricitabine (FTC) and siRNA) in the breast cancer cell line MDA-MB-231.
In the above cell-penetrating cyclic peptides, successive combinations of tryptophan and arginine residues give rise to different types of cell-penetrating cyclic peptides with different cellular delivery properties. These data reveal the molecular transport potential of monocyclic, bicyclic and tricyclic cell-penetrating peptides and provide a good basis for the design of next-generation drug delivery peptides.
Although the molecular design of cell-penetrating peptides is currently reported in the literature in great detail, translating them into the clinical setting remains a formidable challenge. The field of application of cell-penetrating peptides is vast and evolving, and deeper mechanistic exploration has led to an increase in the complexity of molecular design. This includes the development of stable and multi-domain cyclic or self-assembled nanostructures. In addition, further developments are needed in terms of selectivity, targeting and efficiency. Many researchers have proposed controlled spatial folding, cyclisation, dimerisation, stapling, self-assembly and even peptide mimics with different backbones to achieve improved stability and activity of cell-penetrating peptides.
References