In 1989, a number of analogs of lactam cyclized α-MSH/NDP-MSH were created with the aim of creating melanocortin ligands that were more powerful and had a longer duration of action. The active tetrapeptide sequence of NDP-MSH, which consists of His, DPhe, Arg, and Trp, was preserved by cyclizing the truncated ligands through a lactam bridge between positions 5 and 10, as a result of a salt bridge that was thought to exist between the Glu5 and Lys11 of α-MSH/NDP-MSH, according to NMR and computer modeling. Melanotan II (MTII or MT2) is a powerful, non-selective melanocortin ligand that works by truncating three residues from the N- and C-termini, changing Glu5 to Asp5 and Gly10 to Lys10, and then forming a lactam bridge. It acts as an agonist at the MC1R, MC3R, MC4R, and MC5R. Central intracardiac vein injection of MTII inhibited food intake in mice, and this was only one of several in vitro and in vivo applications of MTII since its discovery.
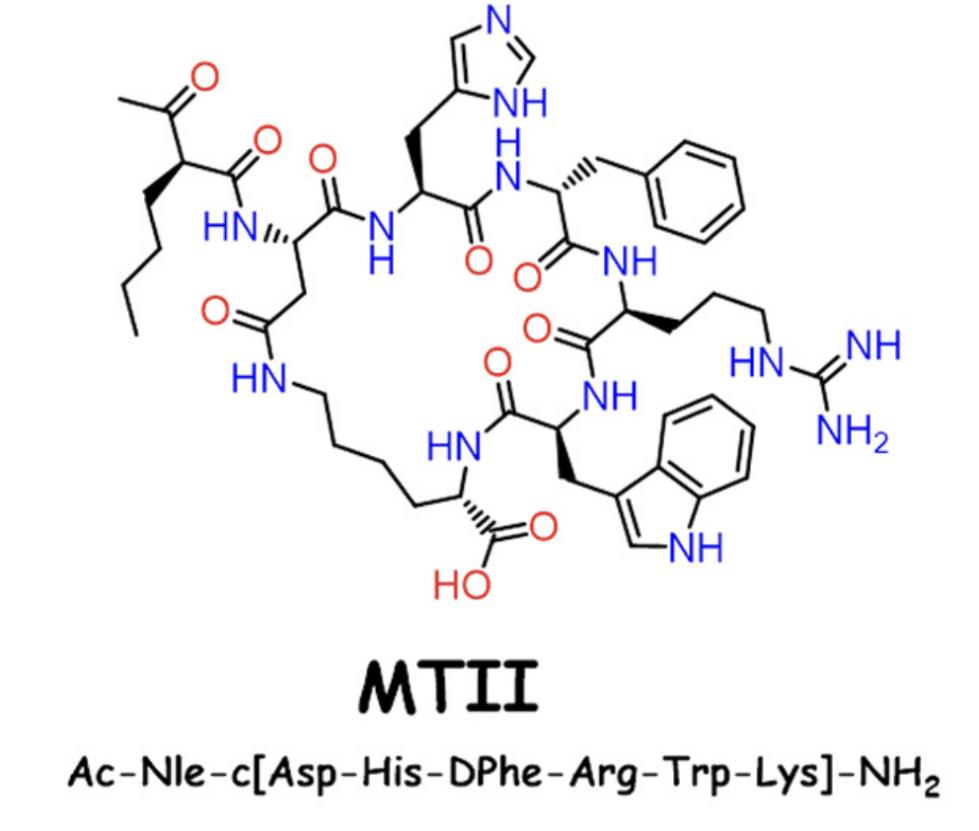 Structure of melanotan II (MTII). (Ericson M D., et al., 2017)
Structure of melanotan II (MTII). (Ericson M D., et al., 2017)
Although their other sequences differ, MTI and MTII share the same His-DPhe-Arg-Trp sequence. MTI ([Nle4-D-Phe7]-MSH) is a 13-amino-acid linear peptide in which norleucine replaces methionine at position 4 and D-phenylalanine stands in for L-phenylalanine at position 7. Its receptor, mainly MC1R, receives a stronger activation from this molecule since it is more resistant to degradation than its natural analogue. With a lactam ring, MT2 (Ac-Nle-[Asp-His-D-Phe-Arg-Trp-Lys]-MSH-NH2) is a shortened variant. Due to its lower receptor specificity, this derivative, which is similar to MTI, is linked to higher unfavorable effects, albeit it is more potent than native α-MSH. In addition to increasing libido and suppressing hunger, this molecule also promotes increased melanin formation.
The original purpose of developing MTI and MTII was to study how these compounds affected melanin formation, with the hope of improving the hormone's inherent photoprotective capabilities. The injection of both substances increased melanin levels, and the resulting melanin was eumelanin, which is more protective of cells than pheomelanin. However, as a side effect, MTII could also cause penile erections. Because of this, studies on MTII as a photoprotector came to a halt, and researchers turned their attention to MTI, which is more selective for melanin formation. However, MTII did not disappear; rather, it shifted its focus to erectile dysfunction research. Different areas of development have been achieved by these two compounds:
A number of photoinduced dermatological disorders, such as solar dermatitis, polymorphic light eruption, erythropoietic protoporphyria, and nonmelanoma skin cancer, are being studied in phase 2 and phase 3 clinical trials as a potential photoprotective drug for MTI. It would appear that these trials have produced promising results. In order to treat erectile dysfunction, a nasally administered chemical (PT-141, Palatin Technologies) was used instead of MTII. It was found to have a core mechanism that causes erections, unlike sildenafil, and to produce an erection that lasts regardless of sexual stimulation in 80% of individuals who did not respond to sildenafil. The chemical was never commercialized since it caused high blood pressure in several patients, despite its potential. Its potential use in hemorrhagic shock is currently being studied by the same company.
 Different structures of NDP-MSH (MTI) and MTII. (Hadley M E., et al., 2006)
Different structures of NDP-MSH (MTI) and MTII. (Hadley M E., et al., 2006)
The pharmacokinetic properties of MT-II were assessed by Ugwu's group in rats following an intravenous administration of 0.3 mg/kg using the bioassay and HPLC-UV techniques described. The following pharmacokinetic parameters were obtained from the bioassay method: Clp: 0.3 ± 0.04 L/kg/h (5 ± 0.67 mL/kg/min), t1/2: 0.5 ± 0.1 h, and Vss: 0.2 ± 0.02 L/kg. However, Clp, t1/2, and Vss were measured using the HPLC method and were found to be 0.3 ± 0.1 L/kg/h (5 ± 3.3 mL/kg/min), 1.5 ± 0.5 h, and 0.5 ± 0.1 L/kg, respectively. Regardless, the two experiments did yield results that were very comparable.
Topical MTII has not been definitively shown to have carcinogenic effects. Through in vitro and in vivo experiments with the B16-F10 melanoma model, this work intends to elucidate the anti-neoplastic efficacy and mechanism of MTII. Researchers discovered that whereas MTII had no effect on cell proliferation, it strongly blocked melanoma cells' capacity to migrate, invade, and form colonies. And in mice with preexisting melanoma, topical MTII administration dramatically slowed tumor growth. According to histological research, MTII treatment caused cell death in melanoma tissues and stopped them from growing and transforming. The results of the immunoblot and immunohistochemistry analyses showed that MTII inhibited AKT/nuclear factor kappa B (NFκB) signaling by increasing the quantity of the phosphatase and tensin homolog (PTEN) protein in a dose-dependent manner while decreasing PTEN phosphorylation. Melanoma cells lacking MTII consistently lacked the ability to produce prostaglandin E2 (PGE2) and inhibit cyclooxygenase II (COX-2) expression. Lastly, research on antibody neutralization has shown that the MC1R is essential for the inhibition of melanoma and upregulation of PTEN produced by MTII. Taken together, these findings suggest that MTII inhibits melanoma growth by downregulating COX-2/PGE2 signaling via inducing PTEN overexpression via MC1R. Therefore, a new approach to treating melanoma may be possible with topical MTII therapy.
Melanotan II is available for purchase online and is usually self-administered by people who want to increase skin pigmentation through the cosmetic benefits of MC1R activation. Melanotan II intensifies UV-mediated tanning and activates MC1R to trigger eumelanin production. Due to its nonselective nature and ability to penetrate the blood–brain barrier, melanotan II also activates the other MCRs, which can lead to negative side effects such weariness, appetite loss, and penile erection. Nonetheless, consumers of this "Barbie drug" frequently enjoy the side effects of heightened libido brought on by melanotan II's actions inside the central nervous system and appetite loss that results in weight loss.
Anesthetized rats were studied to determine the impact of MTII, a non-specific agonist of melanocortin receptors, on erections and the possible locations of its action. In a dose-dependent manner, MTII induced erections by shortening the time it took for the first erection to happen when given intravenously (0.1, 0.3, and 1 mg/kg) or intraventricularly (0.1 and 1μg) into the paraventricular nucleus of the hypothalamus. More pronounced erectile events were observed in rats given MTII intramuscularly (0.2μg) at the L6-S1 level compared to rats given the vehicle intramuscularly. Intravenous administration of MTII (1 mg/kg) enhanced erection-inducing responses to stimulation of the cavernous nerve. In contrast, no facilitator activity was observed when MTII (1μg) was administered into the corpus cavernosum. The neurological processes behind the facilitator effect of MTII were examined in this work using acute spinalization (T8 level) and differential selective nerve transections. Spinal cord or bilateral penile nerve transection did not modify the facilitative effect of intravenous MTII (1 mg/kg). Acute ablation of the paravertebral sympathetic chain in the back, however, removed the MTII facilitator effect. The results provide credence to the theory that MTII can affect erection in two ways: as an inducer and a facilitator, depending on the route of administration.
Mice given the melanocortin agonist MT II intraperitoneally have a severe, temporary hypometabolism/hypothermia. It is maintained in mice that do not have any of the 1, 3, 4, or 5 melanocortin receptors, indicating a mechanism that is not dependent on the canonical melanocortin receptors. The mast cells are necessary by showing that MTII-induced hypothermia was eliminated in KitW-sh/W-sh mice, which do not have mast cells. The receptor MRGPRB2 is capable of detecting a wide range of cationic compounds and activating mast cells without the need for an antigen. Both MRGPRB2-dependent and -independent methods were used by MTII to stimulate mast cells in vitro, and MRGPRB2-null animals did not exhibit MTII-induced hypothermia. MTII treatment raised plasma histamine levels in both wild-type and MRGPRB2-null mice, but not in KitW-sh/W-sh animals, confirming that MTII activated mast cells. Because the hypothermia was significantly reduced by either a specific antagonist, pyrilamine, or ablation of H1 receptors, the released histamine caused hypothermia via histamine H1 receptors.
It has been claimed that melanocortins are involved in the regulation of sexual behavior in both men and women. The current study investigated the effects of melanotan-II (MT-II), a cyclic peptide analogue of alpha-melanocyte stimulating hormone, on aspects of female sexual behavior that are consummatory and appetitive. These aspects included receptivity (lordosis) and sexual proceptivity (solicitations, hops and darts, ear wiggling, and pacing). Estradiol benzoate (EB) and progesterone (P) (10 μg and 500 μg, respectively) were primed subcutaneously in one group of ovariectomized Long-Evans rats (n = 7), while EB (10 μg) and oil (EB alone) were primed in another group (n = 7). Males with sexual experience were used in paced mating tests in unilevel chambers that were divided by a Plexiglas barrier with three holes that only the female could pass through. Ten minutes prior to each 30-minute timed mating test, an intravenous injection of MT-II (1 and 3 mg/kg) or saline was administered. The three treatments were given to each female. Both MT-II dosages markedly increased the frequency of hops, darts, and ear wiggling in females primed with EB + P, but they had no effect on pacing or lordosis. MT-II had no effect on any of the characteristics examined when EB was used alone. These findings imply that P can enhance proceptive behaviors by interacting with MT-II. These data imply that activation of melanocortin receptors may represent a promising mode of action for the treatment of women with hypoactive sexual desire, as hops and darts are essentially solicitations, made in close proximity to the male, that indicate a desire on the part of females to receive mounts and intromissions.
In Otsuka Long-Evans Tokushima Fatty (OLETF) rats, the effects of peripheral injection of the melanocortin agonist MTII on insulin sensitivity and glucose tolerance were investigated. OLETF rats' food intake and body weight were reduced when MTII was administered subcutaneously using osmotic mini-pumps. On day 9, insulin tolerance testing revealed that the MTII group was more sensitive to insulin than the pair-fed or free-feeding groups. In glucose tolerance tests on days 11 and 23, the MTII group likewise displayed noticeably lower glucose readings than the ad libitum group. Therefore, MTII improved glucose tolerance and raised insulin sensitivity in OLETF rats.
In a study that used a maternal immune activation (MIA) animal model of autism, the therapeutic potential of MTII was assessed in adult male mice with autistic-like characteristics. Symptoms of autism, including heightened repetitive behaviors, diminished vocal communication, and subpar social behavioral measures, were observed in the male MIA mice. Male MIA mice regained social behavioral measures after seven days of continuous MTII treatment. There was no discernible change in social behavioral assessments in male C57 mice treated with MTII and coming from normal backgrounds. Additionally, normal C57 mice administered MTII underwent substantial weight loss during subacute treatment, but exhibited no alteration in repetitive or anxious-like behaviors. In the adult male MIA mouse model, these data demonstrate that MTII is an effective therapy for behavioral abnormalities resembling autism.
MT II, a potent agonist at the melanocortin receptor, has been investigated for its potential neurotrophic and neuroprotective effects. The neurotrophic properties of MTII were investigated by means of the sciatic nerve crush model. The sensory function recovery of rats following a sciatic nerve crush damage was significantly enhanced by MT II when administered at a dosage of 20 μg/kg every 48 hours. However, doses of 2 or 50 μg/kg had no effect. In addition, MT II partially protected the nerve from cisplatin-induced toxic neuropathy, proving its neuroprotective capabilities. This means that melanotan II, a potent α-MSH analog, is effective in neuroprotection and neuron regeneration, which is recently demonstrated in the data.
PT-141 is the MT II's metabolite, bremelanotide PT-141, it is a homologue of menalocortin. In a placebo-controlled lab research, males with ED who had previously found sildenafil to be beneficial reported that intranasal delivery of PT-141 improved erections in response to visual sexual stimulation (VSS). Penile erections are the result of administering PT-141 to rats and nonhuman primates. Rats administered PT-141 systemically exhibit increased c-Fos immunoreactivity, indicating that the drug stimulates neurons in the hypothalamus. The pseudorabies virus is injected into the corpus cavernosum of the rat penis and is absorbed by neurons in the same area of the central nervous system. When PT-141 was administered to both healthy males and erectile dysfunction patients, erectile activity increased quickly and dose-dependently. According to the findings, PT-141 may be a promising new treatment for sexual dysfunction.
FAQ
1. How to store melanotan II?
Because it is a synthetic peptide, MT II requires proper storage conditions to maintain its efficacy. Keep the lyophilized powder at -20°C in a dark, airtight container away from moisture and light to keep it stable for months or even years. Use within two to four weeks of reconstituting with sterile or bacteriostatic water and keep in the fridge at 2 to 8°C to prevent degradation. Carefully mark the vial with the reconstitution date, treat it with the utmost cleanliness, and avoid subjecting it to repeated freeze-thaw cycles to maintain its sterility and efficacy.
2. How does MT II work?
MT II activates the melanocortin receptors, which are MC 1, MC 3, MC 4, and MC 5. Its clinically noticeable sexual effects are believed to be caused by its capacity to activate the MC 4 receptor, while it is also believed that MC 3 is involved. It generates melanin via activating the MC 1 receptor. Melanocortin II attaches to melanocortin receptors on skin cells, which control the skin's pigment production. It is the synthetic peptide's binding to these receptors that increases melanin synthesis. A possible side effect of using melanotan is a darkening of the skin tone.
Related Peptides at Creative Peptides
| CAT# | Product Name | Molecular Formula | Price |
|---|---|---|---|
| M09001 | a-MSH, amide | C77H109N21O19S1 | Inquiry |
| M09002 | a-MSH, Free Acid | C77H108N20O20S1 | Inquiry |
| M09003 | [Des-Ac] a-MSH, amide | C75H107N21O18S1 | Inquiry |
| M09004 | g-MSH | C74H99N21O16S1 | Inquiry |
| M09005 | g-1-MSH, amide | C72H97N21O14S1 | Inquiry |
| M09006 | MSH Release Inhibiting Factor, amide | C13H24N4O3 | Inquiry |
| M09007 | b-MSH, porcine | C98H138N26O29S1 | Inquiry |
| M09010 | Melanocyte Associated Antigen gp 100 (17-25) | Inquiry | |
| M09011 | Melanotropin-Potentiating Factor, MPF | C13H24N4O3 | Inquiry |
| M09012 | RAB38/NY-MEL-1 (50-58) | Inquiry | |
| R1511 | Melanotan (MT)-II | Inquiry |
References