Oligopeptides are short chains of amino acids that are typically made up of 2-20 amino acid units. They are smaller and less complex than peptides and proteins, which can consist of many hundreds of amino acids. Multiple research studies confirm that proteins are ultimately assimilated in the guise of oligopeptides and amino acids, with the transportation capability of oligopeptide carriers surpassing the combined capacities of various amino acid carriers. Upon entering the human body and transitioning into organic tissues, oligopeptides are defined by their swift absorption, minimal energy expenditure, and low saturation level. Moreover, oligopeptides exhibit numerous novel functions exceeding those of the original protein amino acids. Oligopeptides are integral to the living process, assuming vital roles in the biological operations of the body such as hormone modulation, immune reaction, and cell-to-cell communication. They also have the potential to act as antibiotics. Insulin, oxytocin, and vasopressin serve as examples of oligopeptides.
Dipeptides, tripeptides, tetrapeptides, and oligopeptides are all different types of peptides, which are short chains of amino acids that are linked together by peptide bonds.
These peptides play various roles in the body, from acting as neurotransmitters to regulating various physiological functions. They can also be used in various medical and cosmetic applications. For example, certain peptides are used in anti-aging skin creams for their purported ability to stimulate collagen production, improve skin elasticity, and reduce wrinkles.
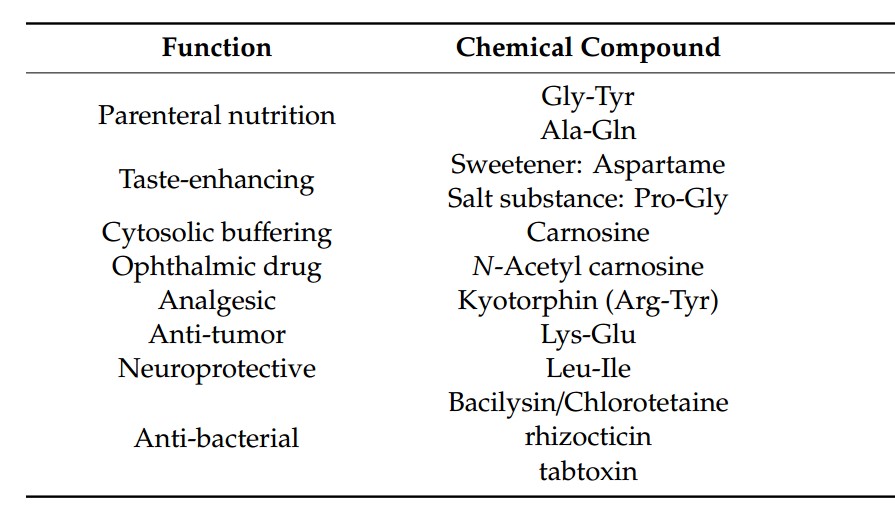 Fig. 1 The dipeptides with interesting biological activities. (Wang, T., 2019)
Fig. 1 The dipeptides with interesting biological activities. (Wang, T., 2019)
Various methods have been reported for the synthesis or formation of peptide bonds, such as chemical synthesis, chemo-enzymatic synthesis, and enzymatic synthesis (biosynthesis).
The chemical synthesis of dipeptides usually includes four principal procedures: (1) protection of functional groups, (2) activation of the free carboxy group, (3) formation of a peptide bond, and (4) removal of the protecting groups. With chemical synthesis, almost all designed dipeptides can be synthesized with appropriate protecting groups, and the yield is usually high.
The SPPS, first introduced by Merrifield, has been a widely used method for oligopeptide synthesis due to its high efficiency and ease of purification. Recent advancements in microwave-assisted SPPS have enhanced the reaction rates, making the synthesis process more cost-effective. However, technical difficulties such as racemization and epimerization remain significant challenges.
As a conventional technique, LPPS offers advantages such as easy scale-up and the ability to prepare large peptides. The recent introduction of heat-assisted LPPS has made it more efficient and environmentally friendly. Yet, the separation of the target peptide from by-products and excess reagents is still a problem.
The combination of LPPS and SPPS, although in its infancy stage, is making a promising approach for oligopeptide synthesis. This method offers the advantages of both SPPS and LPPS, potentially overcoming their individual drawbacks.
The chemo-enzymatic synthesis of dipeptides results from the reverse reaction catalyzed by peptide bond-hydrolyzing enzymes (proteases or esterases). This method includes two distinct types of reaction processes: the thermodynamically controlled process or the kinetically controlled process. The former is carried out to drive the equilibrium toward peptide synthesis with necessary interventions, such as the precipitation of synthesized dipeptides or reaction with a large excess of substrates. The latter is dependent on an acylated serine or cysteine protease, which will then undergo a competitive deacylation process with water and the other amino acid. This method leads to temporary accumulation of the formed dipeptide. Compared with the chemical synthesis process, these two methods usually involve stricter stereoselectivity and milder conditions.
Various enzymatic machineries that can catalyze the synthesis of dipeptides, such as the ribosome, non-ribosomal peptide synthetases (NRPSs), ATP (Adenosine triphophate)-grasp enzymes, and α-amino acid ester acyltransferases, have been found in nature. These naturally occurring peptide-synthesizing enzymes seem to be ideal catalysts for dipeptide synthesis, even though they are diverse in their specificity and physiological function. Based on the intermediates formed in the catalytic process, either aminoacyl-AMP (Adenosine monophosphate) or aminoacyl phosphate, these enzymes are generally divided into two categories The representatives of the former category are the ribosome, tRNA-dependent ligases, adenylate-forming amide ligases, and the NRPSs. The latter category usually contains a variety of enzymes, which usually shares a characteristic ATP-grasp motif in their amino acid sequence and are known as the ATP-grasp enzymes. This includes glutathione synthetase, d-alanine-d-alanine ligase, and other ATP-grasp enzymes.
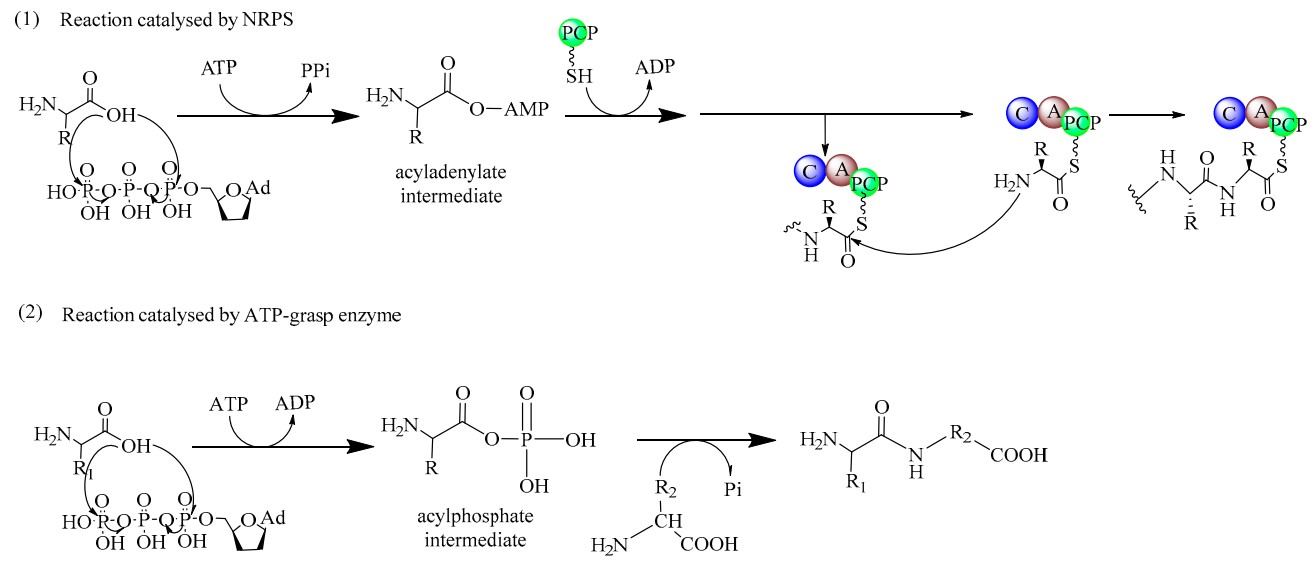 Fig. 2 The reaction mechanism of two distinct types of biocatalysts for the synthesis of dipeptides. (1) Acyladenylate intermediates are formed in the process catalyzed by NRPSs. (2) Acylphosphate intermediates are formed in the process catalyzed by ATP-grasp enzymes. (Wang, T., 2019)
Fig. 2 The reaction mechanism of two distinct types of biocatalysts for the synthesis of dipeptides. (1) Acyladenylate intermediates are formed in the process catalyzed by NRPSs. (2) Acylphosphate intermediates are formed in the process catalyzed by ATP-grasp enzymes. (Wang, T., 2019)
In recent years, with the development of biotechnology and the introduction of oligopeptide absorption mechanism, the research on oligopeptides has received more and more attention. The advantages are mainly reflected in:
Pharmaceuticals: Oligopeptides are used in the pharmaceutical industry in the development of new drugs. They can act as active pharmaceutical ingredients (APIs) and have been used in treatments for conditions like diabetes, cancer, and infectious diseases.
Cosmetics: In the cosmetics industry, oligopeptides are used in different skincare products. They can help with skin rejuvenation by stimulating collagen production, reducing wrinkles, and improving skin elasticity.
Chemical Research: Oligopeptides play a vital role in chemical research, particularly in the study of protein structure and function. They can also be used to study peptide–protein interactions, important for understanding biological processes.
Diagnostics: Oligopeptides can be used in the development of diagnostic tools. They can act as biomarkers in different diagnostics tests, helping to detect diseases in the early stages.
Vaccines: Some oligopeptides have been used in the development of vaccines. They can help stimulate an immune response, leading to the production of antibodies against specific pathogens.
Food Industry: In the food industry, oligopeptides are used as functional foods because of their health-enhancing properties. They can also be used in the preparation of dietary supplements.
Biotechnological Applications: Oligopeptides can be used in various biotechnological applications like enzyme immobilization, cellulose degradation, and in the advancement of bioactive materials.
Agriculture: Certain oligopeptides have been explored for their potential uses in plant growth and protection, by increasing the resistance of plants to pests or improving their adaptation to environmental stress.
Wound Healing: Some oligopeptides have shown promising results in promoting wound healing because of their antimicrobial properties and their ability to stimulate cell migration and growth.
Fitness Supplements: Oligopeptides are used in the creation of certain bodybuilding and fitness supplements due to their ability to help in muscle recovery and growth.
Every step of peptide synthesis is subject to Creative Peptides' stringent quality control. Typical delivery specifications include:
Oligopeptide synthesis is a scientific technique used to create oligopeptides, which are short chains of amino acids. it allows precise control over the sequence of the peptides, and is often used in pharmaceutical development, biochemical research and life science studies.
The process of oligopeptide synthesis involves the agglomeration of amino acids in a specific order. This is primarily achieved through techniques such as solid-phase peptide synthesis (SPPS) or liquid-phase peptide synthesis (LPPS).
As a leading biopharmaceutical company, we have advanced technologies and expertise that allow us to synthesize a wide range of oligopeptides. However, the feasibility of synthesizing certain peptides depends on their complexity and characteristics.
Yes, our synthesis services are highly customizable. We can synthesize peptides based on the specific needs and requirements of our client. In addition to oligopeptide synthesis, we also provide services such as peptide modification, labeling, and purification.
Yes, we do provide oligopeptide synthesis for bulk orders. We have a well-equipped facility and experienced professionals who can accommodate large-scale production while still maintaining high-quality standards.
We utilize state-of-the-art analytical technologies such as HPLC and MS for quality control to ensure the purity of our oligopeptides. Each synthesized oligopeptide undergoes stringent quality checks before delivery.
The cost of our oligopeptide synthesis service varies depending on several factors such as the complexity of the peptide sequence, the scale of synthesis, the level of purity required, and additional modifications or services. Please contact us for a customized quote.

References