2017-05-25
Basic information
Desmopressin is a synthetic analogue of the antidiuretic hormone vasopressin used in the treatment of central diabetes insipidus and nocturnal ensuresis and as a haemostatic agent. The selective antidiuretic activity is due to its ability to bind to only V-2 receptors and not to V-1 receptors. V-2 receptors are G-protein coupled receptors present in the collecting ducts of the kidney and are responsible for promotion of water reabsorption via stimulation of cyclic AMP production.
| CAT# | 10-101-11 |
| Product Name | Desmopressin |
| CAS No. | 16679-58-6 |
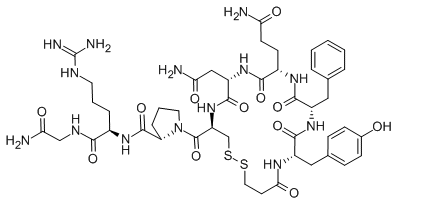
| Sequence | 3-Mercaptopropionyl-Tyr-Phe-Gln-Asn-Cys-Pro-D-Arg-Gly-NH2 |
| M.W/Mr. | 1069.2 |
| Molecular Formula | C46H64N14O12S2 |
Medical uses of desmopressin
Clinically, desmopressin is used for the treatment of diabetes insipidus and nocturnal enuresis. It is also used as a haemostatic drug in patients with mild haemophilia A, von Willebrand’s disease or platelet dysfunction due to its ability to cause release of endogenous factor VIII, von Willebrand factor and tissue plasminogen activator into the blood circulation. It is usually administered orally or intranasally, however the bioavailability of the peptide is only 0.1% for the oral route and 3.4% for the nasal route in humans. The poor bioavailability is due largely to its low lipophilicity, but degradation of the peptide by enzymes in the gut limen and intestinal and nasal mucosal tissues may also play a role. In rats, the oral bioavailability has been shown to be even lower than human and the least among the different species tested. The major reason attributed to the low bioavailability was poor permeability properties of the rat intestinal mucosa to the hydrophilic drug. Various prodrug strategies have been applied to desmopressin to improve its bioavailabilty.
Desmopressin as a model of peptide drug
The interest in peptides as potent drugs increased dramatically in the last two decades due to recent advances in synthetic and molecular-biology techniques, enabling large-scale manufacture of these substances. However, with very few exceptions of small and cyclic peptides such as cyclosporin, most peptide drugs have low oral bioavailabilities with typical values of less than 1%. This phenomenon results mainly from the poor permeability of the intestinal mucosa to high molecular-weight peptides; the extensive proteolytic degradation of most peptides, both high and low molecular-weight by digestive enzymes in the gastrointestinal (GI) tract; the insufficient closeness of the drug or delivery system to the absorbing intestinal mucosa and its short residence time at the GI absorption site. Consequently, most news peptide-based drugs are administered via the parenteral route, a route which is not well accepted by patients, particularly for chronic therapy.
Desmopressin was chosen as a model of peptide drug to be modified using cell penetrating peptides (CPPs) for the following reasons. Firstly, it is a low molecular weight peptide with an inherent disulfide bond, which can be used to ligate CPPs. It also has a tyrosine residue that can be radiolabelled with 125I to trace the molecule. Secondly, as mentioned above, it has low permeability and poor bioavailability when administered orally and intranasally, therefore any enhancement in the permeability and increase in bioavailability due to conjugation with CPP will be clearly evident. Furthermore it is relatively cheap and a safe drug with a wide therapeutic window.
Bioreversible derivatization of desmopressin to give more lipophilic prodrug forms may be a useful approach to enhance its bioavailability. It was shown that esterification of the tyrosine phenolic group in α-N-acylated tyrosine amide model compounds improved the stability of the tyrosine amide bond toward hydrolysis by the pancreatic enzyme α-chymotrypsin. The esters are readily hydrolyzed by plasma and liver esterases with formation of the parent peptide. These findings inspired us to apply this prodrug approach to desmopressin.
References:
Kahns, Anne H., Anders Buur, and Hans Bundgaard. "Prodrugs of peptides. 18. Synthesis and evaluation of various esters of desmopressin (dDAVP)." Pharmaceutical research 10.1 (1993): 68-74.
Patel, Leena. Cationic cell penetrating peptides: Characterization of transport properties in epithelial cells and their utilization as delivery systems for protein and peptide drugs. University of Southern California, 2007.