Glycopeptide antibiotics (GPAs) are drugs of last resort for treating severe infections caused by Gram-positive pathogens such as Staphylococcus aureus (SA), Enterococcus spp., and Clostridium difficile. They remain the front-line therapy for infections caused by methicillin-resistant S. aureus (MRSA), which is presently the most important cause of antimicrobial-resistant hospital infections worldwide. GPAs are also used to treat healthcare-associated infections caused by Enterococcus faecalis and Enterococcus faecium, which are naturally resistant to other classes of antibiotics and easily acquire additional resistance through horizontal gene transfer or mutation.
(vancomycin and teicoplanin) They are natural metabolites produced by the filamentous actinomycetes Amycolatopsis orientalis and Actinoplanes teichomyceticus, respectively. Their common scaffold is a nonribosomal heptapeptide made by proteinogenic and nonproteinogenic amino acids (five aromatic amino acids in vancomycin and all seven aromatic in teicoplanin) tailored with sugar residues, chlorine atoms, methylgroups, and - in the case of teicoplanin - a lipid chain. Cross-linking of aromatic residues (three in vancomycin and four in teicoplanin) confers the peculiar structural conformation that represents the binding pocket for the antibiotic target in bacterial cell walls. GPAs inhibit peptidoglycan (PG) synthesis by binding to the D-alanyl-D-alanine (D-Ala-D-Ala) terminus of the peptide stem of PGprecursor lipid II. Binding of GPAs to lipid II by forming five hydrogen bonds locks PG precursors, impeding subsequent cross-linking reactions. The mechanism of resistance - which has been extensively studied in vancomycin resistant enterococci (VRE) - consists in replacing D-Ala-D-Ala by D alanyl-D-lactate (D-Ala-D-Lac) (in VanA and VanB phenotypes) or D alanyl-D-serine (D-Ala-D-Ser) (VanC), thus reducing the antibiotic affinity for its molecular target.
They are semi-synthetic derivatives of natural products, where the modifications were introduced outside the D-Ala-D-Ala binding pocket, mainly involving the appendage of hydrophobic aryl or acyl groups that mimic the natural lipid chain of teicoplanin. Telavancin is a derivative of vancomycin that differs by the addition of a hydrophobic decylaminoethyl side chain on the vancosamine amine and a hydrophilic (phosphomethyl)aminoethyl group on the C-terminal aromatic ring. Oritavancin is the chlorobiphenylmethyl semi-synthetic derivative of chloroeremomycin, which is a vancomycin-family natural scaffold variant produced by A. orientalis, only differing from vancomycin in the pattern of glycosylation. Dalbavancin is the dimethylaminopropyl amide derivative at the C-terminal of the teicoplanin-family natural scaffold A40926 produced by the actinomycete recently reclassified as Nonomuraea gerenzanensis. The superior antimicrobial potency of these molecules is due to membrane anchoring of the hydrophobic tail, which strengthens the bond to membrane-localized lipid II and might promote back-to-back dimerization of GPAs. Additionally, telavancin and oritavancin cause membrane depolarization and increase permeability, thus enhancing activity.
Studies on the structure of vancomycin provided a foundation for the determination of all other glycopeptide antibiotics. Early attempts to elucidate the structure of vancomycin were hampered by impurities, lack of crystallinity, and structural complexity. As improvements in purification methods and newer spectroscopic techniques came along, the pioneering studies on the structure of vancomycin became possible. Building on these studies, Harris established the complete structure of vancomycin in 1982. Soon to follow were the full structural characterizations of ristocetin and teicoplanin. In 1995, the first crystallographic analysis of an intact, naturally occurring glycopeptide antibiotic, balhimycin, was reported by Sheldrick et al. Soon thereafter followed the crystal structures of vancomycin and the parvodicin aglycon.The structure of vancomycin bound to a surrogate ligand (acetate) was solved by X-ray crystallographic techniques in 1997 and in 1998 the structure of vancomycin bound to N-acetate-d-alanine was solved, which supported the proposed biological mechanism of action. Based on these landmark achievements, the structures of hundreds of natural and semisynthetic glycopeptides have been, and are currently being, determined with relative ease. These structures are highly related and fall within five structural sub-types, I-V.
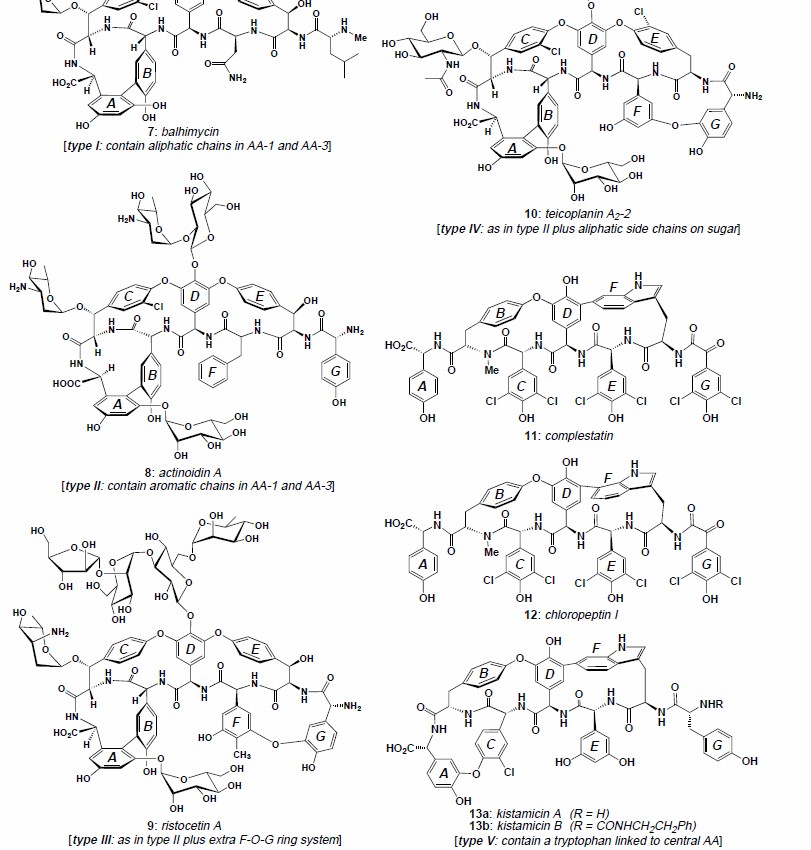 Structural types of glycopeptide antibiotics
Structural types of glycopeptide antibiotics
The large majority of the GPAs known today were discovered by natural product screening of soil actinomycetes collected around the world in the pre-genomics era.
GPAs are industrially produced in low-cost rich and complex media, which contain slow-releasing carbon, nitrogen, and phosphorous sources. Chemically defined minimal media have sometime been employed to evaluate the specific effect of carbon and nitrogen sources, phosphate, ammonium, and calcium on GPA productivity. To limit the effect of catabolite repression, the fed-batch process, in which one or more nutrients are added in order to control the growth rate according to their concentration, is the process preferred for producing GPA at the industrial scale since the growth phase can be prolonged without inhibiting secondary metabolite production.
With the advent of DNA sequencing and cloning technologies, biochemical pathways of GPAs started to be inferred largely from sequence analyses. BGCs for GPAs include genes physically clustered on the chromosome, which encode for the nonribosomal peptide synthetase (NRPS) assembling the heptapeptide, for tailoring enzymes (oxidation, glycosylation, chlorination, methylation, sulfation, and acylation), for precursor biosynthetic pathways, for cluster-situated regulators, transporters and /or resistance factors.
Our Glycopeptide Antibiotics
| CAT# | Product name | CAS No. | Price |
|---|---|---|---|
| Z10-101-160 | Dalbavancin Impurity (A40926) | 102961-72-8 | Inquiry |
| 10-101-103 | Vancomycin | 1404-90-6 | Inquiry |
| 10-101-104 | Teicoplanin | 61036-62-2 | Inquiry |
| 10-101-78 | Dalbavancin | 171500-79-1 | Inquiry |
| MFP-076 | Balhimycin | 140932-79-2 | Inquiry |
References