Amino acids are carbon, hydrogen, oxygen, nitrogen, and sulphur atoms organic molecules. They're the structural units of proteins, and of many major molecules in life. There is usually an amino group (NH2), a carboxyl group (COOH), and a side chain (R-group), and these properties make amino acids amphoteric, i.e. they are zwitterions at physiological pH.
Three amino acids are called essential amino acids (EAAs), nonessential amino acids (NEAAs) and conditional amino acids. Important amino acids: essential amino acids are those which aren't produced by humans in the body, and have to be consumed through food. non-essential amino acids: can be formed through the metabolism of other molecules in the body. Conditionally essential amino acids: may have to be consumed through diet for certain special conditions like during infancy or disease states.
In the organism, amino acids do many different things including but not limited to:
Protein synthesis: Amino acids are linked by peptide bonds to form peptide chains, which are then folded into proteins that perform various biological functions.
Neurotransmitters: Certain amino acids such as glutamate and glycine act as neurotransmitters and are involved in the transmission of nerve signals.
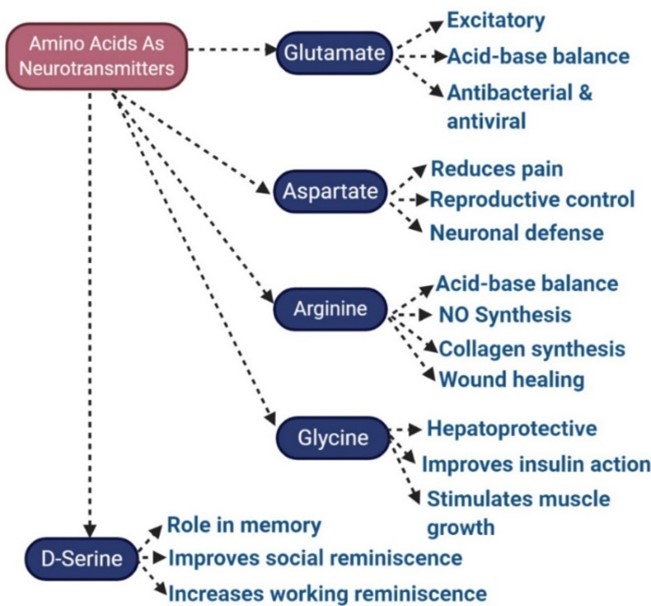 Fig.1 Amino acids involved in nerve signaling. (Gasmi, Amin, et al., 2022)
Fig.1 Amino acids involved in nerve signaling. (Gasmi, Amin, et al., 2022)
Metabolic regulation: Amino acids are involved in the metabolic process of carbohydrates and fats, helping to maintain energy balance.
Precursors to hormones and enzymes: Some amino acids are precursors to hormones and enzymes, such as tyrosine, which is a precursor to thyroid hormones.
Immune function: Amino acids are essential for the proper functioning of the immune system, and a deficiency of certain amino acids may lead to a decrease in immunity.
Differences in Types of Amino Acids
Amino acids are organic molecules that are the building blocks of proteins and serve crucial roles in various metabolic pathways and physiological processes. Based on different criteria, they can be classified in several ways to better understand their roles in life processes. The main classifications of amino acids include those based on nutritional necessity, side chain properties, structure, function, and hydrophobicity. Below, we will provide a simple overview of how amino acids can be classified into these different categories and their key characteristics.
Table.1 Overview of Amino Acid Classifications.
| Classification | Key Feature |
|---|
| Based on Nutritional Needs | Amino acids are classified based on whether the body can synthesize them or if they need to be obtained from the diet. |
| Based on Side Chain | Amino acids are categorized based on the chemical nature of their side chains, affecting their interactions with proteins. |
| Based on Structure | Amino acids are classified according to the structure of their side chains (acidic, basic, neutral, simple, etc.), impacting protein function. |
| Based on Function | Amino acids are classified by their roles in physiological processes like structural support, signaling, energy metabolism, and immune function. |
| Based on Hydrophobic Properties | Amino acids are categorized based on whether their side chains are hydrophilic (water-attracting) or hydrophobic (water-repelling), influencing protein solubility and structure. |
Classification of Amino Acids Based on Nutritional Needs
Amino acids are organic compounds that serve as the building blocks of proteins and play various roles in metabolism. They can be classified based on the body's ability to synthesize them and their necessity in different physiological conditions:
Essential Amino Acids (EAAs)
Essential amino acids are amino acids that cannot be produced by the human body and must be obtained through the diet.
- Lysine is a basic amino acid necessary for protein synthesis, calcium absorption, and tissue repair.
- Tryptophan is an aromatic amino acid that acts as a precursor for serotonin and niacin, influencing mood and sleep.
- Phenylalanine is an aromatic amino acid involved in synthesizing tyrosine and neurotransmitters like dopamine and norepinephrine.
- Methionine is a sulfur-containing amino acid essential for methylation reactions and antioxidant defense.
- Threonine is a hydroxyl-containing amino acid critical for collagen and elastin formation and immune function.
- Isoleucine is a branched-chain amino acid vital for muscle repair and energy production.
- Leucine is a branched-chain amino acid important for muscle protein synthesis and energy metabolism.
- Valine is a branched-chain amino acid essential for nitrogen balance and muscle tissue repair.
- Histidine is an amino acid required in infancy for growth and is involved in hemoglobin and histamine production.
Semi-essential Amino Acids (SEAAs)
Semi-essential amino acids are synthesized by the body but may require dietary supplementation during growth, illness, or stress.
- Arginine is a conditionally essential amino acid that supports wound healing, immune function, and nitric oxide production.
- Histidine also falls into this category for adults under certain physiological conditions, serving roles in enzyme function and neurotransmission.
Non-essential Amino Acids (NEAAs)
Non-essential amino acids are those that the body can produce and do not need to be obtained from food.
- Glycine is a simple amino acid involved in collagen synthesis and neurotransmission.
- Alanine is a non-polar amino acid that plays a role in energy metabolism and glucose synthesis.
- Serine is a polar amino acid important for metabolic pathways, including nucleotide and lipid synthesis.
Conditionally Essential Amino Acids (CEAAs)
Conditionally essential amino acids are typically non-essential but become necessary in certain conditions like illness or stress.
- Proline is a conditionally essential amino acid important for collagen production and tissue repair.
- Glutamine is the most abundant amino acid in the body, supporting gut health and immune function during stress.
Classification of Amino Acids Based on Side Chain
Classifying amino acids by their side chain properties allows us to better understand the chemical properties of amino acids and their role in protein structure and function. For example, non-polar side-chain amino acids tend to aggregate inside proteins to form a hydrophobic core, while polar uncharged side-chain amino acids tend to be located on the surface of proteins and form hydrogen bonds with water molecules. Amino acids can be classified according to the nature of their side chains, which are mainly divided into the following categories:
Non-polar side-chain amino acids
These amino acids typically have side chains composed of carbon and hydrogen, are uncharged, and are highly hydro phobic. Common non-polar side-chain amino acids include glycine (Gly), alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), and tryptophan (Trp).
- Glycine is the simplest amino acid with a hydrogen atom as its side chain, contributing to flexibility in protein structures.
- Alanine is a non-polar amino acid with a methyl group as its side chain, involved in energy metabolism and glucose synthesis.
- Valine is a branched-chain amino acid with an isopropyl group in its side chain, essential for muscle repair and nitrogen balance.
- Leucine is a branched-chain amino acid with a sec-butyl group in its side chain, crucial for muscle protein synthesis and energy metabolism.
- Isoleucine is a branched-chain amino acid with a sec-butyl group in its side chain, vital for muscle tissue repair and energy production.
- Proline is a non-polar amino acid with a cyclic side chain, playing a key role in collagen stability and protein structure.
- Phenylalanine is an aromatic amino acid with a benzyl side chain, essential for neurotransmitter synthesis and mood regulation.
- Tryptophan is an aromatic amino acid with an indole group in its side chain, important for serotonin production and regulating sleep and mood.
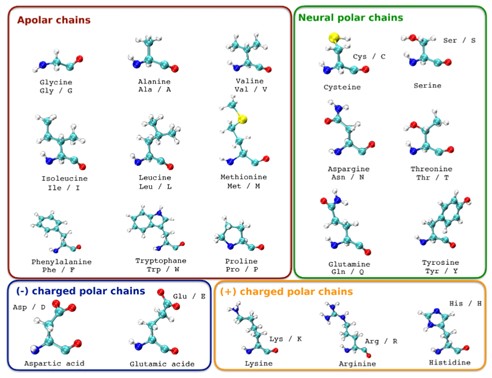 Fig.2 Amino acids classified according to the polarity of their side chains. (Pellegrino, Eric, et al., 2023)
Fig.2 Amino acids classified according to the polarity of their side chains. (Pellegrino, Eric, et al., 2023)
Table.2 Amino acid related services at Creative Peptides.
Polar uncharged side chain amino acids
The side chains of these amino acids contain polar groups (such as hydroxyl groups, sulfhydryl groups, etc.) but are not charged. Common polar uncharged side-chain amino acids include serine (Ser), threonine (Thr), cysteine (Cys), asparagine (Asn), glutamine (Gln), and tyrosine (Tyr).
Serine is a polar amino acid that contains a hydroxyl group in its side chain and is important for enzyme activity and protein function.
- Threonine is a polar amino acid with a hydroxyl group, essential for protein phosphorylation and enzyme regulation.
- Cysteine is a polar amino acid containing a sulfhydryl group, involved in forming disulfide bonds that stabilize protein structures.
- Asparagine is a polar amino acid that contains an amide group, playing a role in protein synthesis and post-translational modifications.
- Glutamine is a polar amino acid with an amide group, important for nitrogen transport and supporting immune function.
- Tyrosine is a polar amino acid with a hydroxyl group on its aromatic ring, playing a key role in hormone synthesis and cell signaling.
Positively charged side-chain amino acids
The side chains of these amino acids are positively charged under physiological conditions and often contain amino or imidazole groups. Common positively charged side-chain amino acids include lysine (Lys), arginine (Arg), and histidine (His).
- Lysine is a positively charged amino acid with an amino group in its side chain, crucial for protein structure, gene expression, and enzymatic functions.
- Arginine is a positively charged amino acid that contains a guanidinium group, important for nitric oxide production, wound healing, and immune function.
- Histidine is a positively charged amino acid with an imidazole group, essential for enzyme catalysis, particularly in hemoglobin and histamine production.
Negatively charged side-chain amino acids
The side chains of these amino acids are negatively charged under physiological conditions and usually contain carboxyl groups. Lesser negatively charged side-chain amino acids include aspartic acid (Asp) and glutamic acid (Glu).
- Aspartic acid is a negatively charged amino acid containing a carboxyl group, important for protein interactions, enzyme catalysis, and neurotransmission.
- Glutamic acid is a negatively charged amino acid with a carboxyl group, playing a key role in neurotransmission and cellular metabolism.
Aromatic side-chain amino acids
The side chains of amino acids are made up of aromatic rings like phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp).
- Phenylalanine is an aromatic amino acid with a benzyl side chain, involved in synthesizing tyrosine and neurotransmitters like dopamine.
- Tyrosine is an aromatic amino acid with a hydroxyl group on its benzene ring, involved in neurotransmitter synthesis and as a precursor to thyroid hormones.
- Tryptophan is an aromatic amino acid containing an indole side chain, important for producing serotonin, a neurotransmitter that regulates mood and sleep.
Heterocyclic side chain amino acids
The side chains of these amino acids contain other atoms besides carbon, hydrogen, oxygen, nitrogen, such as histidine (His) and proline (Pro).
- Histidine is a heterocyclic amino acid containing an imidazole group, which is involved in enzyme catalysis and plays a critical role in hemoglobin function.
- Proline is a heterocyclic amino acid with a unique cyclic side chain, contributing to the structure and stability of proteins, particularly in collagen.
Classification of Amino Acids Based on Structure
Amino acids can be classified into the following categories based on their chemical structure:
Acid amino acids
Side chains of these amino acids have carboxyl groups, commonly aspartic acid and glutamic acid.
- Aspartic acid is an acidic amino acid with a carboxyl group in its side chain, playing a key role in protein-protein interactions and neurotransmission.
- Glutamic acid is an acidic amino acid containing a carboxyl group, important for cellular metabolism, neurotransmitter function, and maintaining the pH balance in the body.
Basic amino acids
This group of amino acids has an amino group in its side chain, commonly Lysine, Arginine, and Histidine.
- Lysine is a basic amino acid that contains an amino group in its side chain, essential for protein structure, gene regulation, and enzyme functions.
- Arginine is a basic amino acid with a guanidinium group in its side chain, crucial for nitric oxide production, wound healing, and immune function.
- Histidine is a basic amino acid with an imidazole group, involved in enzyme catalysis, acid-base balance, and hemoglobin function.
Neutral amino acids
The side chains of such amino acids are neither positively nor negatively charged, and the common ones are glycine, proline, etc.
- Glycine is a neutral amino acid with a simple hydrogen atom as its side chain, important for protein flexibility and collagen formation.
- Proline is a neutral amino acid with a unique cyclic side chain, contributing to protein stability, especially in collagen and structural proteins.
Simple amino acids
Amino acids with no additional functional groups. Glycine (Gly), for example, is the simplest amino acid and is found in a wide variety of proteins.
- Glycine is a simple amino acid with no additional functional groups, and it is the simplest amino acid, commonly found in proteins, particularly collagen.
Hydroxyl Amino Acids
The side chain contains the hydroxyl group and is polar. Serine (Ser) and threonine (Thr) are often involved in signal transduction and protein modification.
- Serine is a hydroxyl amino acid with a hydroxyl group in its side chain, important for protein phosphorylation and enzyme activity.
- Threonine is a hydroxyl amino acid with a hydroxyl group, essential for protein modification, signal transduction, and collagen formation.
Sulfur-containing amino acids
Side chains contain sulfur atoms, forming disulfide bonds. For example, cysteine (Cys) plays a key role in protein folding and stabilization. Methionine (Met) is a promoter of many metabolic processes.
- Cysteine is a sulfur-containing amino acid with a sulfhydryl group in its side chain, playing a key role in the formation of disulfide bonds, which stabilize protein structures.
- Methionine is a sulfur-containing amino acid, essential for initiating protein synthesis and promoting many metabolic processes, including methylation reactions.
Classification of Amino Acids Based on Function
Structure-related amino acids
Involved in the formation of bio-macromolecular structures. For example, glycine (Gly) and proline (Pro) play a major role in collagen, enhancing the strength of connective tissue.
- Glycine is a structure-related amino acid that plays a major role in collagen formation, enhancing the strength and flexibility of connective tissue.
- Proline is a structure-related amino acid that is critical for collagen synthesis and stability, contributing to the structural integrity of connective tissues.
Signal transduction amino acids
Such as tyrosine (Tyr), are precursors to thyroid hormones and neurotransmitters.
- Tyrosine is a signal transduction amino acid that serves as a precursor to thyroid hormones and neurotransmitters, playing a vital role in regulating mood and metabolism.
Energy metabolizing amino acids
Glutamine (Gln), as an alternative source of cellular energy, is particularly important in metabolic stress.
- Glutamine is an energy-metabolizing amino acid that serves as an alternative source of energy during metabolic stress, supporting cellular function and tissue repair.
Immunomodulatory amino acids
Such as histidine, are involved in the immune response. Arginine (Arg) can promote immune cell function and enhance wound healing.
- Histidine is an immunomodulatory amino acid involved in immune response, participating in the synthesis of histamine and supporting the body's defense mechanisms.
- Arginine is an immunomodulatory amino acid that enhances immune cell function and promotes wound healing through nitric oxide production.
Classification of Amino Acids Based on Hydrophobic Properties
Hydrophilic amino acids: Such as serine, threonine, tyrosine, etc., the side chain groups of these amino acids can form hydrogen bonds with water molecules.
- Serine is a hydrophilic amino acid whose side chain contains a hydroxyl group, allowing it to form hydrogen bonds with water molecules, contributing to protein solubility.
- Threonine is a hydrophilic amino acid with a hydroxyl group in its side chain, enabling it to interact with water and play a key role in protein structure and function.
- Tyrosine is a hydrophilic amino acid that has a polar hydroxyl group in its side chain, facilitating hydrogen bonding with water and its involvement in signal transduction.
Hydrophobic amino acids: Such as glycine, alanine, valine, etc., the side chain groups of these amino acids are more hydrophobic.
- Glycine is a hydrophobic amino acid with a small side chain, making it non-polar and allowing it to be tucked into protein interiors to maintain structural stability.
- Alanine is a hydrophobic amino acid with a methyl group in its side chain, which makes it non-polar and favors its placement in the hydrophobic core of proteins.
- Valine is a hydrophobic amino acid with a branched-chain side group, contributing to its non-polar nature and its preference for the interior of proteins, away from aqueous environments.
Summary
As an important part of living organisms, amino acids are classified in various ways and have their own characteristics. Understanding the different types of amino acids and their functions is important for studying fields such as biochemistry, medicine, and nutrition. Through the introduction of this paper, readers can better understand the role of amino acids in living organisms and their classification methods, so as to provide reference for research in related fields.
References
- Gasmi, Amin, et al., Neurotransmitters regulation and food intake: The role of dietary sources in neurotransmission. Molecules 28.1 (2022): 210.
- Pellegrino, Eric, et al., Extreme Gradient Boosting Tuned with Metaheuristic Algorithms for Predicting Myeloid NGS Onco-Somatic Variant Pathogenicity. Bioengineering 10.7 (2023): 753.
 Fig.1 Amino acids involved in nerve signaling. (Gasmi, Amin, et al., 2022)
Fig.1 Amino acids involved in nerve signaling. (Gasmi, Amin, et al., 2022)  Fig.2 Amino acids classified according to the polarity of their side chains. (Pellegrino, Eric, et al., 2023)
Fig.2 Amino acids classified according to the polarity of their side chains. (Pellegrino, Eric, et al., 2023)