2017-05-25
Somastotatin (SST) is a hormone that inhibits growth, initially identified by Brazen et al. in 1973 within the hypothalamus of animals. Structurally, SST exists in two forms, both featuring a short half-life of less than 3 minutes. SST is broadly distributed throughout the central and peripheral nervous systems, as well as the gastrointestinal tract. Additionally, it has been detected in the hearts of rats and humans, and within the mucosa of the gastrointestinal tract. SST is released by D cells, with its highest concentration found in the antrum and gastric body.
Somatostatin (SS) is an endogenous peptide hormone isolated, purified and characterized in 1973 from almost 500,000 sheep hypothalamic fragment. The molecule is a 14-amino acid cyclic peptide, which is an important regulatory peptide hormone, with a broad range of activities. The amino acid sequence of somatostatin was determined by Edman degradation. The peptide was synthesized by solid phase techniques and the biological activity of the synthesized peptide was determined to be identical to that of native (ovine) somatostatin. Later, somatostatin was synthesized by several other groups and its biological activities examined. Gerich et al. Observed in 1974 that somatostatin produced inhibition of postprandial elevations in plasma glucose, glucagon and growth hormone (GH) in juvenile (insulin-dependent) diabetes. This observation sparked the interest of numerous researchers, because of the potential for treatment of diabetes.
There are two forms of native somatostatin, somatostatin-14 (SS-14), a 14-amino acid cyclic peptide, and somatostatin-28 (SS-28), which differs from somatostatin-14 by a fragment of 14 amino acids at the amino terminus of SS-14. Both biologically active peptides are encoded by a single gene, and their biosynthesis has been elucidated. Both molecules are disulfide-bridged. They derive from the enzymatic cleavage of a large, 92-amino acid biologically inactive precursor, prosomatostatin (Pro-SS), which in turn arises from preprosomatostatin, an 116 amino acid molecule. Pro-SS is subjected to tissue-specific processing, such that SS-14 and SS-28 are differentially represented in various tissues. Depending on the location, different cell types produce differing amounts of both peptides. Pancreatic δ cells, neural cells and peripheral neurons predominantly produce SS-14, while intestinal mucosal cells predominantly produce SS-28 and account for the largest peripheral source of this peptide. A novel gene was identified, called Cortistatin (CST) gives rise to two gene products similar to SS, CST-17 and CST-29. However, the expression of CST has so far been shown to be restricted to the cerebral cortex.
The secretion of SS can be modulated by a wide variety of stimuli including ions, neurotransmitters, neuropeptides, growth factors and hormones. Some of the agents that stimulate secretion of SS act directly in non-specific manner while others appear to tissue selective in their action or act indirectly through other transmitters to effect SS release. The release of SS from pancreatic cells as well as from neurons occurs following membrane depolarization and an influx of calcium suggesting a common mode of release irrespective of cell type. The effect of nutrients such as glucose and amino acids have been studies in the hypothalamus and the gut. Hypothalamic SS secretion is abrogated by glucose. However, amino acids have no effect on the release. In the pancreas, glucagon and arginine stimulates release of SS pointing to potential regulators of SS secretion in the pancreas. In general, glucagon, neurotensin, bombesin, growth hormone releasing hormone stimulate the release of SS from various sites while opiates and γ-amino butyric acid inhibit secretion of from various sites and tissues. A number of cytokines appear to modulate the secretion as well as synthesis of SS from immune cells. Interferon-γ, and Interleukin-10 have been shown upregulate SS expression in macrophages. In normal breast tissue SS immunoreactivity to detected only in the stroma, while epithelial cells show SS production only after malignant transformation.
SST is an evolutionarily conserved cyclic peptide, existed in two molecular forms (SST-14,SST-28) with disulfide bonds between cysteine residues maintaining its cyclic structure. Both of the two forms are proteolytically hydrolyzed from the same precursor (92 peptide somatostatin precursor). Somatostatin-14 shares the same 14 amino acids as the carboxy-terminus of somatostatin-28. The biological activity of somatostatin-14 and somatostatin-28 is present in the cyclic region of mature peptides. SST-14 has a strong inhibitory effect on glucagon and gastrin. Whereas SST-28 focuses on suppressing growth hormone and insulin.
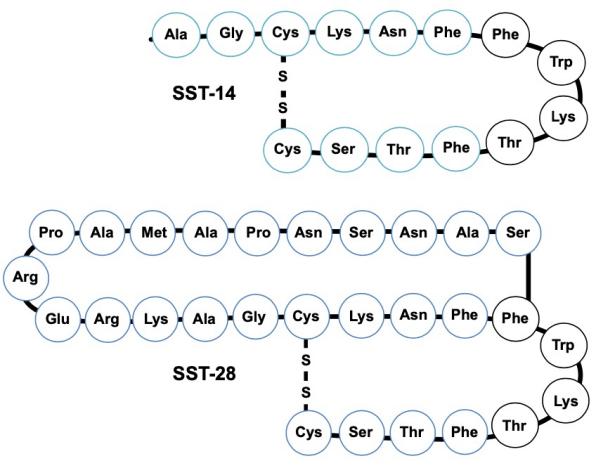 Somatostatin structure ( Cuevas-Ramos D. 2016)
Somatostatin structure ( Cuevas-Ramos D. 2016)
Somatostatin structure. The two somatostatin isoforms in humans are depicted in schematic diagram. Somatostatin is a cyclical peptide of 14 (SST-14) or 28 (SST-28) amino acids.
Somatostatin possesses the ability to inhibit the secretion of various hormones, including growth hormone, thyroid-stimulating hormone, insulin, and glucagon. It also inhibits gastric acid secretion that is stimulated by test meals and 5-peptide gastrin, as well as the release of pepsin and gastrin. Furthermore, somatostatin regulates gastrointestinal absorption and nutritional function. Additionally, it exhibits a significant capacity to decrease visceral blood flow, portal venous pressure, blood flow and pressure in collateral circulation, and hepatic blood flow. It also reduces the internal and external secretion of the pancreas, as well as the secretion of the gastroenteric small intestine and gallbladder. Moreover, somatostatin decreases enzyme activity and exhibits a protective effect on pancreatic cells.
Somatostatin and its analogues exhibit specific binding to somatostatin receptor (SSTR) located on the cellular surface, thereby activating SSTR. Somatostatin exerts an inhibitory influence on cellular proliferation by different signaling pathways. Additionally, somatostatin indirectly suppresses the proliferation of tumor cells by inhibiting homones and releated factors that promote cells growth. Hormones that somatostatin can inhibit encompass growth hormone (GH), endothelial cell growth factor (EGF), cholecystokinin (CCK), and insulin-like growth factor-1 (IGF-1), among others. Furthermore, somatostatin promotes the apoptosis of tumor cells, leading to cell cycle arrest and retaining tumor cells in the G0-G1 phase. It also possesses immunomodulatory properties, enhancing the activity of phagocytes, lymphocytes in rats, and human natural killer (NK) cells.
Somatostatin is able to improve cognitive function in the brain, improving visual processing and cognitive behavior by reducing excitatory input from neurons in the cortex. In the central nervous system, somatostatin modulates neurotransmission and memory formation.
The effect of somatostatin depends on its interaction with specific receptors. Somatostatin receptors (SSTRs) are an important class of G protein-coupled receptors (GPCRs), which play a key role in regulating the secretion of various hormones and inhibiting tumor growth. So far, it has been found that there are five subtypes of somatostatin receptors (SSTR1-SSTR5), which are mainly distributed in organs and tissues such as the digestive tract, pancreas, and adrenal glands. Somatostatin is a natural ligand of SSTRs, which has the effect of inhibiting the secretion of various hormones such as growth hormone, insulin, and glucagon. SST inhibits the activity of adenylate cyclase (AC) by binding to SSTRs, which is mainly mediated by inhibitory G protein (Gi), which then regulates the intracellular cAMP concentration, transmits exogenous signals to the cell, and affects the secretion of various hormones and tumor growth.
Researchers have provided a detailed elucidation of the binding mode and activation mechanism of the endogenous polypeptide SS-14 and its receptor, at the atomic level. The endogenous polypeptide SS-14 occupies the orthostatic binding pocket of the receptor composed of TM2-7 and ECL2-3. The receptor occupies the part of F-W-K-T that requires a ring-like structure. The conserved motif "W8-K9" of the peptide is inserted inside the 7TM of the receptor, and the N- and C-terminus of the polypeptide linked by disulfide bonds (Cys3-Cys14) are oriented towards the extracellular.
Native SST is not effective in clinical applications owing to its extremely short half-life of one to three minutes, as it undergoes rapid degradation by peptidases that are ubiquitous in plasma and tissues. Following the characterization of SST, various synthetic analogs of SST (SSAs) with prolonged half-lives have been developed. These analogs are intended to replicate the effects of somatostatin in vivo, primarily by inhibiting the secretion of various other hormones, including growth hormone, insulin, and glucagon. Given their capacity to suppress hormone secretion, somatostatin analogs are employed in the treatment of multiple medical conditions, such as acromegaly, neuroendocrine tumors, and severe diarrhea associated with certain gastrointestinal disorders. Their precise mechanism of action entails binding to somatostatin receptors located on the surface of target cells, thereby regulating cellular activity and hormone production.
The initial synthetic somatostatin analog (SSA) is octreotide, a synthetic octapeptide mimic of human somatostatin. Octreotide demonstrates a strong affinity for somatostatin receptor subtype 2 (SSTR2) and inhibits the proliferation of cells expressing the SSTR2 gene by activating the tyrosine phosphatase signaling pathway. Lanreotide, a cyclic octapeptide developed in the 1990s with the aim of creating a more prolonged acting SSA, along with octreotide, are categorized as first-generation SSAs. Pasireotide, belonging to the second-generation of SSAs, exhibits a higher affinity for SSTR1 (30-fold), SSTR3 (5-fold), and SSTR5 (39-fold), and an equivalent affinity for SST2 (three-fold) in comparison to octreotide.
Somatostatin & Analogs at Creative Peptides
| CAT# | Product Name | M.W | MF | Price |
|---|---|---|---|---|
| 10-101-169 | Pasireotide | 1047.20624 | C58H66N10O9 | Inquiry |
| 10-101-26 | Octreotide Acetate | 1019.24 | C49H66N10O10S2 | Inquiry |
| 10-101-44 | Vapreotide Acetate | 1191.44 | C59H74N12O11S2 | Inquiry |
| M34140635H | [Nal3]Octreotide acetate | 1069.3 | C53H68N10O10S2 | Inquiry |
| M34140636H | TETA-Octreotide acetate | 1433.7 | C67H96N14O17S2 | Inquiry |
| M34140637H | NOTA-Octreotide trifluoroacetate | 1304.5 | C61H85N13O15S2 | Inquiry |
| M34140643H | DOTA-Lanreotide acetate | 1481.7 | C70H95N15O17S2 | Inquiry |
| M34140653H | [Tyr3,Lys5(Boc)]octreotide acetate | 1135.36 | C54H74N10O13S2 | Inquiry |
| M34140655H | [Lys5(Boc)]lanreotide acetate | 1196.44 | C59H77N11O12S2 | Inquiry |
| O1003 | ([ring-D5]Phe3)-Octreotide | 1024.3 | C49H61D5N10O10S2 | Inquiry |
| S07018 | (D-2-Nal5,Cys6·11,Tyr7,D-Trp8,Val10,2-Nal12)-Somatostatin-14 (5-12) amide | 1192.47 | Inquiry | |
| S07041 | Tyr-(D-Dab4,Arg5,D-Trp8)-cyclo-Somatostatin-14 (4-11) | 1276.51 | Inquiry | |
| S07043 | Vapreotide | 1131.39 | Inquiry |
Somatostatin inhibitors, on the other hand, function by blocking the action of somatostatin, which can lead to increased hormone secretion. This class of drugs is particularly useful in conditions where the suppression of somatostatin activity is beneficial, such as in certain types of acromegaly or neuroendocrine tumors. By inhibiting somatostatin, these drugs can stimulate the release of growth hormone or other hormones that are normally suppressed by somatostatin, thus offering a therapeutic approach that complements the effects of somatostatin analogues.
Somatostatin and its analogues exhibit inhibitory effects on gastrointestinal bleeding, alleviate severe conditions associated with acute pancreatitis, and notably enhance the cure rate of pancreatitis. Furthermore, they demonstrate a substantial inhibitory influence on digestive tract tumors, including gastrointestinal neuroendocrine tumors and liver metastases.
Somatostatin significantly improves the therapeutic outcomes of diabetic ketoacidosis and diabetic retinopathy. In recent years, it has also demonstrated favorable efficacy in the treatment of diabetic nephropathy. Additionally, somatostatin inhibits the synthesis and release of various endocrine inflammatory mediators and cytokines, exerting immunosuppressive effects. Consequently, it is utilized in the management of hyperthyroidism.
References