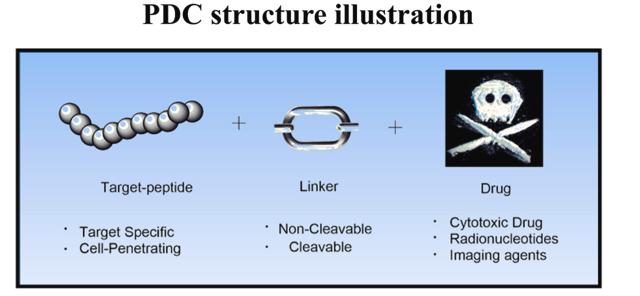
Peptide-drug conjugate (PDC) is a targeted therapeutic agent that is similar in structure and function to antibody-drug conjugate (ADC). It is made up of connections between different types of peptides and drugs. PDC consists of three important components: peptides, linkers and cytotoxic drugs. These three parts coordinate the delivery of chemotherapy drugs by targeting receptors on tumor cells to expand their therapeutic effect.
 Structure of peptide-drug conjugate (Fu C, et, al. 2022)
Structure of peptide-drug conjugate (Fu C, et, al. 2022)
In PDC drugs, the main role of peptides is to achieve targeting. The selection of peptides can affect the endocytosis efficiency of PDC drugs, and has significant effects on pharmacokinetics, efficacy and therapeutic index. There are two types of peptides commonly used in PDC: One is cell-targeting peptides, including PEGA, somatostatin analogues, bombesin analogues, RGD peptides, etc.; The second is cell penetrating peptides, such as Pep-1, Pentratin, PepFact14, and Transportan.
Peptides and small molecules have significantly different pharmacokinetic properties. Among them, the biggest disadvantage of peptide drugs is their low bioavailability and drug uptake, and peptides are usually not able to be administered orally. Therefore, the rapid renal clearance and short half-life hamper the in vivo study of the peptide, as well as factors affecting its drug-forming properties. There are several ways to improve the ADMTE properties of peptides:
1) Increase cell permeability.
2) Enhance chemical stability and anti-proteolytic ability.
3) Reduce renal clearance rate and prolong circulation half-life.
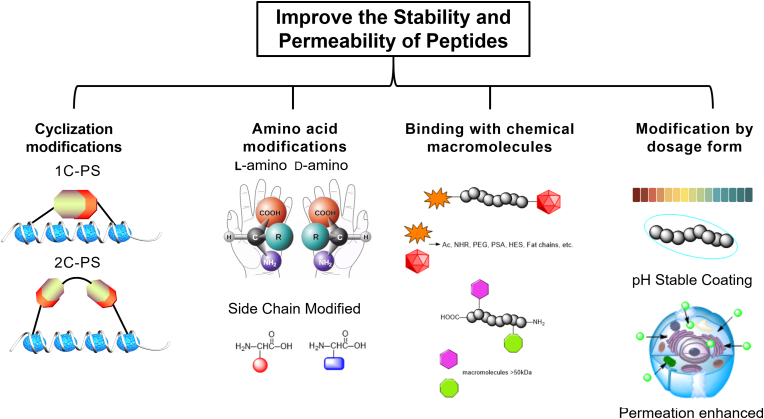
At present, the common strategies to improve the stability and cell permeability of peptides mainly include the following:
1) Cyclization of peptides. Cyclization is widely used in peptide synthesis, including head-to-tail cyclization, side chain cyclization, side chain and side chain cyclization. Peptide anastomosis is often used to determine the secondary structure of the peptide, such as α-helix and β-folding, etc., which can improve the binding affinity of the peptide to the target and increase its ADME.
2) Amino acid modification. Another way to increase the stability of the peptide is to use D-configuration amino acids instead of L-configuration amino acids. This reduces the amino acid sequence, substrate recognition, and binding affinity of proteolytic enzymes.
3) Modifications combined with chemical macromolecules. The charge of the peptide is associated with renal clearance. Negatively charged peptides have a longer half-life than positively charged peptides. Peptides with higher molecular weight (>450 kDa) can increase the lipophilicity of the peptide. In addition, modification methods such as PEG modification, PSA modification, HES modification, fat chain modification and other modifications can also increase the half-life of peptides.
4) Change the dosage form. Intracellular protein delivery systems typically rely on the fusion of genetic proteins with penetrating membrane tags and protein-encapsulated carriers based on cationic liposomes, polymers, and inorganic nanomaterials. It has been reported that there are several ways to enhance the oral bioavailability of peptide therapeutics through dosage forms, such as the addition of penetration enhancers and acid-resistant coatings, etc.
 Different methods used to improve the stability and permeability of peptides (Fu C, et, al. 2022)
Different methods used to improve the stability and permeability of peptides (Fu C, et, al. 2022)
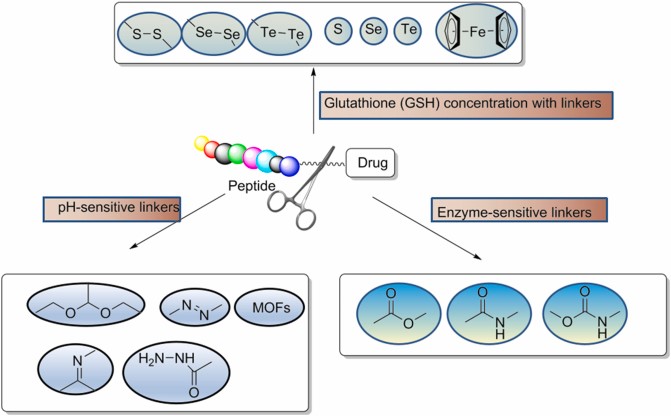
The linker is designed to allow sufficient cycle time for the drug to reach its target cell. The selection of linkers is one of the key factors in the design of PDC, and it is necessary to consider the microenvironment in which the PDC is located so as not to interfere with the binding affinity and drug efficacy of the peptide and its receptor. Depending on its length, stability, release mechanism, functional group, hydrophilic/hydrophobic properties, different types of linkers are used in PDC. The linker used in PDC must demonstrate stability to prevent premature and non-specific drug release. The linker of PDC drugs is no different from the linker of ADC. As with ADCs, linkers for PDC can be cleavable (esters, amides, and carbamates) or non-cleavable (thionates, oximes, and triazoles).
 The chemical structures of different linker (Fu C, et, al. 2022)
The chemical structures of different linker (Fu C, et, al. 2022)
Toxic drugs are an integral part of the process of killing tumors. After PDC enters the cell, the toxic drugs are the factors that finally lead to the death of the target cell. Therefore, the toxicity and physicochemical properties of toxic drugs can directly affect the ability of drugs to kill tumors, thus affecting their efficacy. In general, cytotoxins must have the following four requirements: clear mechanism of action, small molecular weight, high cytotoxicity, and chemical conjugation with peptides to maintain anti-tumor activity. However, each toxic drug usually has its own limitations, such as poor PK properties, etc. PDC payloads also involve a variety of types, including nuclides, MMAE, docetaxel (TH1902), DNA topoisomerase inhibitors (SN-38, CBX-12), and PARP inhibitors.
The structure of PDC is similar to that of ADC, the difference is that the targeting unit of ADC is an antibody, and PDC is a peptide. The mechanism of action of PDC is also similar to that of ADC. Through the decomposing link chain in the cell, the targeting peptide and cytotoxin are covalently linked to accurately target specific receptors of tumor cells, and the cytotoxin is released in a controlled manner, thus killing tumor cells.
| Property | PDC | ADC |
|---|---|---|
| Molecular weight | The weight of the molecule (2–20 kDa) is small, which makes it easier to penetrate the tumor stroma and enter the tumor cells | Large molecular weight (~160 kDa) limits passive transport through epithelial cell membranes |
| Pharmacokinetic | Rapidly eliminated by the kidneys, making it less toxic to the bone marrow and liver | Non-specific uptake by the liver and reticuloendothelial system results in dose-limiting toxicity to the liver and bone marrow |
| Cost | It can be expressed in situ or chemically synthesized for simple production and easy scale-up | Relatively difficult to manufacture and costly to produce and qualify |
| Cytotoxic payload | Targeted formulations can be coupled with a variety of clinically proven cytotoxic molecules such as adriamycin, paclitaxel, camptothecin, cisplatin, and so on | Cytotoxic molecules are limited to a very few highly toxic candidates such as MMAE (monomethyl auristatin E), and DM-1 (mertansine) |
1. Peptides are easier to synthesize and purify
Compared with monoclonal antibodies, peptides are easier to synthesize and purify, which makes the production and transportation costs of PDC drugs lower.
2. Easy modification of peptide structure
Reduced difficulty for drug design, resulting in improved bioavailability, binding affinity, and stability.
3. The molecular weight of peptide drugs is smaller than that of antibodies, and the penetration is strong
Peptide drugs have a molecular weight between small molecules and biological products, which makes it easier to penetrate the tumor matrix and enter the tumor cells.
4. The structure and composition are simpler and have lower immunogenicity
PDC has lower immunogenicity, that is, the probability of generating immune stress response in the body is lower.
5. Higher security
PDC can be eliminated by the kidneys and is less toxic to the liver.
6. PDC drugs can be developed for a wide variety of indications
The indications of PDC mainly include esophageal tumors, brain tumors, metastatic non-small cell lung cancer, stomach tumors, ovarian tumors, multiple myeloma, pancreatic tumors, and advanced solid tumors.
The first PDC drug is Lutathera, a subsidiary of Novartis, for the treatment of a type of cancer of the pancreas or gastrointestinal tract, known as gastroenteropancreatic neuroendocrine tumors (GEP-NETs). This variety belongs to an emerging class of peptide receptor radionuclide therapies that work by binding to a type of cell called a somatostatin receptor, which may be present on some tumors. After binding to the receptor, the drug enters the cell, where it is irradiated to damage the tumor cell.
Novartis's peptide nuclide conjugate lutetium Lu 177 vipivotide tetraxetan can be listed as a listed PDC drug, which is a 177Lu-labeled radiopharmaceutical for the treatment of PSMA-positive castration-resistant prostate cancer that has progressed after treatment. The trade name is Pluvicto.
Reference