The half-life of a drug determines how long it stays in the body. Short half-lives can be compensated by dosing intervals and dosages, but there are limitations in terms of cost and safety. Those peptide drugs that have received regulatory approval provide us with successful examples of improving peptide stability from minutes to hours or more. The plasma half-life of approved peptide therapeutics is significantly improved compared to endogenous peptides. Peptide chemistry uses different modification strategies to prolong the stability of peptide therapeutics. These chemical modifications are used to stabilize bioactive peptides and alleviate hematological or cathepsin mediated degradation and inactivation, thereby enhancing the pharmacotropic properties of peptide candidates.
N-terminal acetylation of peptides is one of the most common means of chemical modification of peptides to increase their stability. It is worth noting that as of the end of 2021, 37 entities out of 62 marketed short peptides (composed of less than or equal to 15 amino acid residues) (excluding the withdrawn romulpeptides and ambamustine) have been closed in their n-terminal by acylation, pyroglutamate or cyclization. Among the Nα unblocked peptide drugs, 11 are disulfide bond-containing cyclic peptides and 1 amideated cyclic peptide, and one is prodrug. From the analysis of the data, only nine of the short peptide drugs approved by the FDA by the end of 2021 actually have a free Nα group.
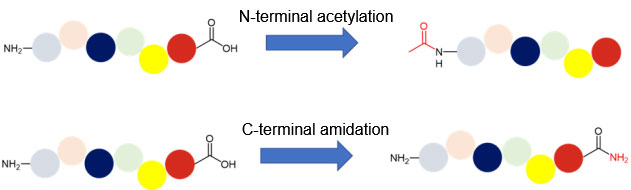 N-terminal acetylation and C-terminal amidation of peptides
N-terminal acetylation and C-terminal amidation of peptides
In addition to avoiding the enzymatic hydrolysis of exopeptidase, the N-terminal block of the peptide is also an important reason to prevent the chemical degradation of the peptide in the form of DKP (diketopiperazine). Peptide chains with free Nα groups can be degraded by DKP under alkaline to neutral conditions to produce a six-membered ring 2,5-diketopiperazine and the corresponding N-terminal dipeptide shortened peptide chain.
Similarly, amidation can be used at the C-terminal of the peptide to convert the natural carboxylic acid group at the C-terminal of the peptide to the corresponding amide group, reducing the enzymatic hydrolysis of carboxypeptidase for some specific amino acids at the C-terminal of the peptide. If these C-terminal amino acids are replaced with the corresponding amino acid amides, the enzymatic hydrolysis of carboxypeptidase may be significantly reduced.
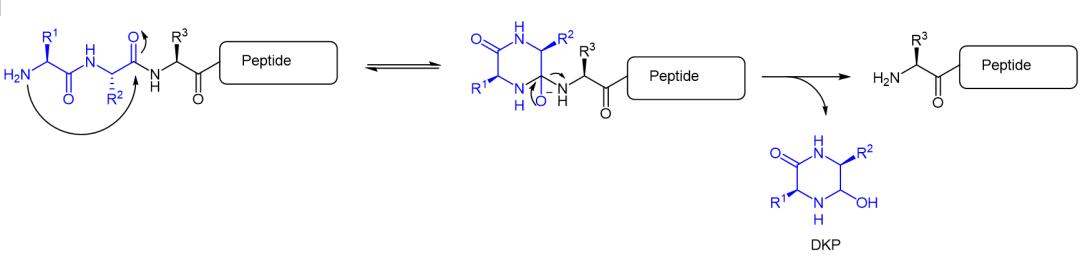 Degradation mechanism of peptide DKP
Degradation mechanism of peptide DKP
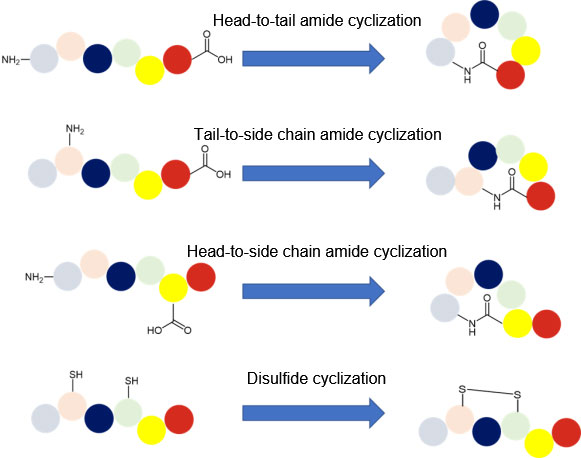 Peptide cyclization
Peptide cyclization
Peptide cyclization is a common modification of peptides. It not only eliminates amino and carboxyl groups at the N-terminal and C-terminal of the peptide which are easily degraded by exopeptidase, but also keeps the configuration of peptides in a state conducive to binding with receptors, thus greatly improving the biological activity of these peptides. The latest FDA-approved new drug, Rezafungin, an Echinalcin peptide analogue for the treatment of candidiemia and invasive candidiasis, is a cyclic peptide drug.
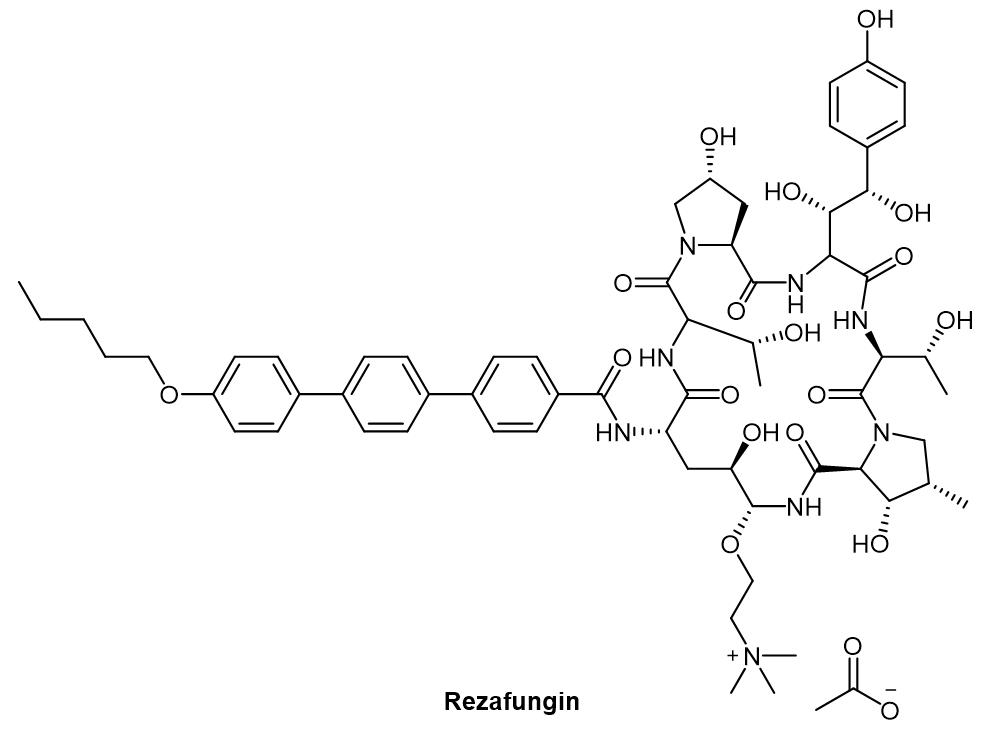 Structure of Rezafungin
Structure of Rezafungin
D-amino acid substitution is also a common means to increase the drug stability of peptides, which can reduce the enzymatic hydrolysis dependent on the spatial conformation. Because ribosomes are specific to L-amino acids, D-peptides rarely occur naturally in living organisms and are not easily digested or degraded. D-peptide mimetics are D-peptides designed to mimic natural L-peptides that often have therapeutic properties. D-amino acid peptides play an important role in the development of peptide antibiotics.
N-methylated peptides can not only increase the chemical stability of peptides and reduce the enzymatic degradation of endopeptidases, but also play a key role in the spatial structure of peptides. Through N-methylation, the affected amide bond can no longer be an H-bond donor, which will have a profound effect on the secondary structure of the peptide. N-methylation has therefore become the most commonly used peptide screening tool for medicinal chemists after Alanine scan and D-amino acid scan.
The famous N-methylated peptide drugs on the market, including cyclosporine A, contain 7 N-methyl amino acids in its structure, which not only greatly improves the hydrolytic stability of cyclosporine, but also forms the basis for its spatial structure. The subtle adjustments that are almost impossible to replicate make cyclosporine the first peptide drug to achieve oral delivery.
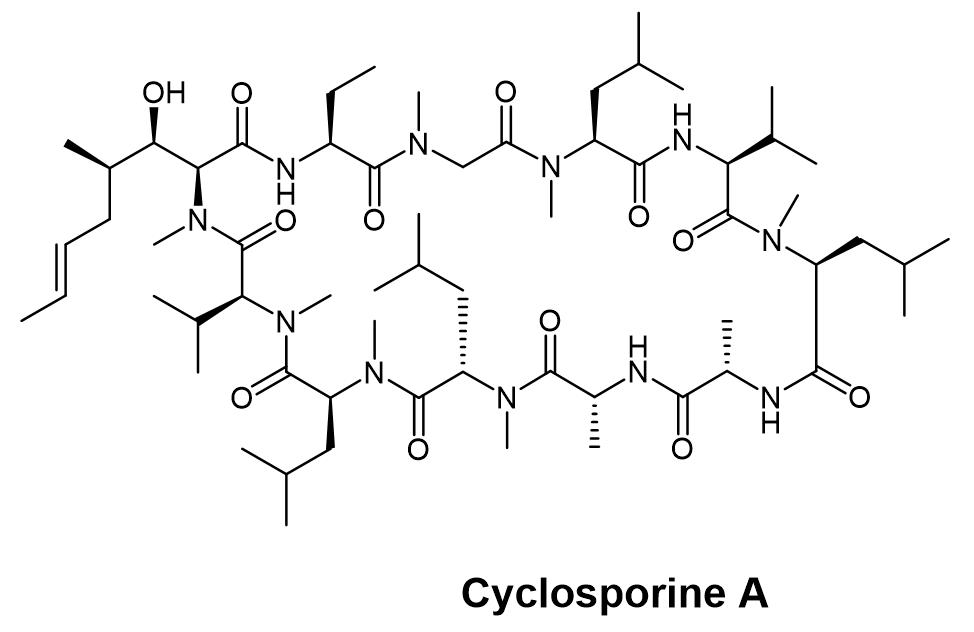 Structure of Cyclosporine A
Structure of Cyclosporine A
Lipidation usually reduces the positive charge of a peptide (the modified amino acid is usually lysine). But more critically, the introduced lipid structure greatly increases the half-life of the peptide, which is generally thought to be achieved through increased binding to albumin. For example, GLP-1 peptide analogs, semaglutide for type 2 diabetes and obesity, and the GLP-1/GIP receptor dual agonist tilpotide, which is also a type 2 diabetes peptide drug. Semaglutide has been developed and marketed orally on the basis of permeation enhancer SNAC.
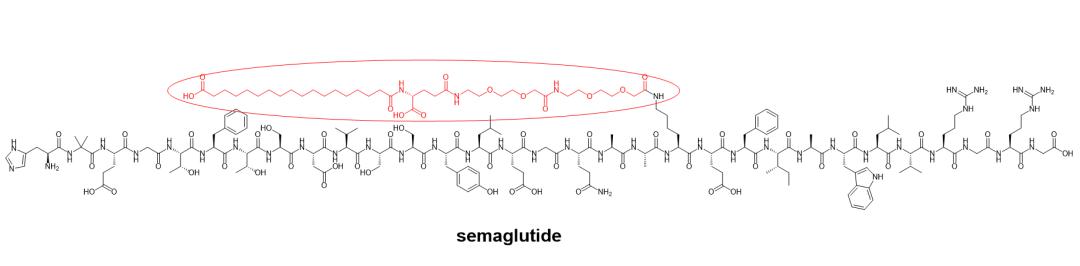 Structure of Semaglutide
Structure of Semaglutide
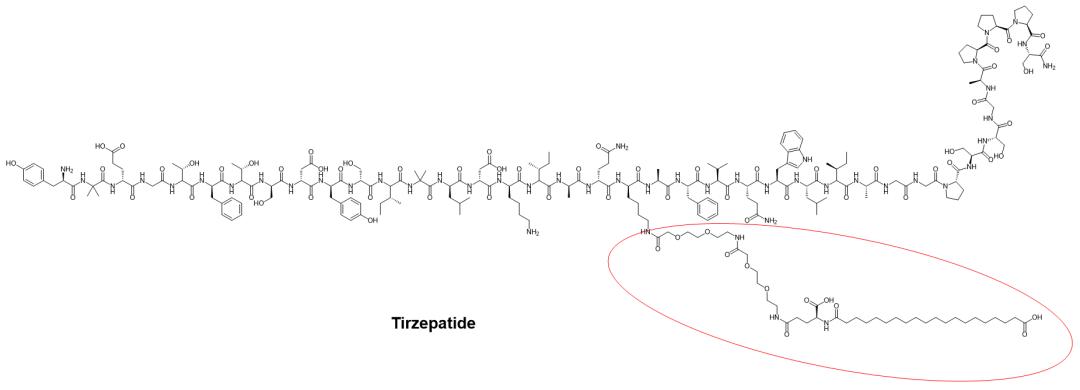 Structure of Tirzepatide
Structure of Tirzepatide
While the vast majority of peptides target extracellular proteins, in order to reach intracellular target receptors, peptides needs to cross the cell membrane. However, peptides usually have only limited passage through this biological barrier. In order to customize peptides for intracellular delivery, conjugating with the CPPs (Cell-Penetrating Peptides) sequence makes it possible to deliver bioactive peptides to the intracellular target. Several cell-penetrating peptides were used in the study, such as penetratin, polyarginine, transporter and TAT (trans-activator of transcription) peptides.
Molecular grafting is a promising trend in peptide drug development and novel probe design. This approach is used to design peptide therapies with drug-like properties. Conformationally restricted and highly stable peptides are used as scaffolds in this strategy, "fusing" with bioactive epitopes to produce what is known as molecular grafting. After synthesizing the probe and verifying its conformational integrity, the grafted peptide is selected based on its biological activity. The resulting molecules combine the advantages of increased stability provided by peptide scaffolds with functionalization provided by bioactive epitopes.
Several naturally derived peptides have been explored for this application, including cyclic cysteine knot peptide, lasso peptide, cyclic sunflower trypsin inhibitor 1, and θ-defensin. The method is used to prepare new probes that target GPCRS, integrins, ion channels, and other cell surface receptors, including vascular endothelial growth factor receptor (VEGFR), enzyme, or protein-protein interactions.
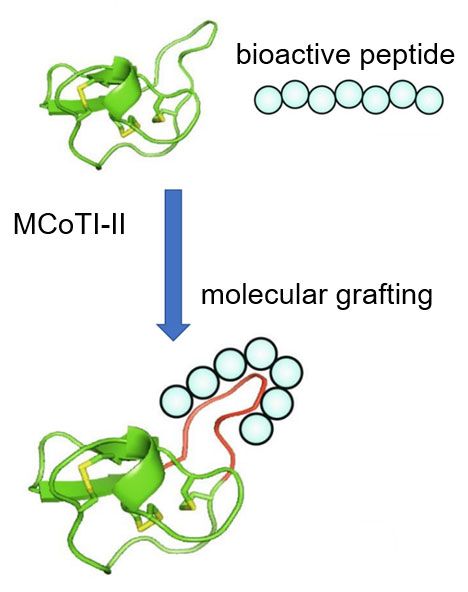 Peptide Molecular Grafting
Peptide Molecular Grafting