Currently a candidate therapy for obesity, cagrilintide is a stable lipidated non-selective long-acting amylin analog given subcutaneously weekly. Based on salmon calcitonin, this dual amylin and calcitonin receptor agonist (DACRA) has shown more effectiveness in obesity treatment, fasting glucose control, and HbA1c management in rat models than in amylin treatment. The important part the calcitonin receptor plays in glucose metabolism might help to explain this efficiency.
Cagrilintide incorporates 14E/17R mutations anticipated to stabilize the central helix through a salt bridge, 25P/28P/29P mutations analogous to rat amylin to diminish β-sheet propensity and fibril formation, a C-terminal proline to enhance potency on CTR specifically, and an N-terminally linked C20 fatty acid to ensure prolonged in vivo presence. The lipidation of cagrilintide and the implemented changes have not diminished its effectiveness compared to amylin, as shown by rat food consumption experiments. Phase 1/2 studies indicate that cagrilintide, either alone or in conjunction with semaglutide, has potential effectiveness in the treatment of obesity.
 Structures of Selected Relevant Peptides. (Kruse T., et al., 2021)
Structures of Selected Relevant Peptides. (Kruse T., et al., 2021)
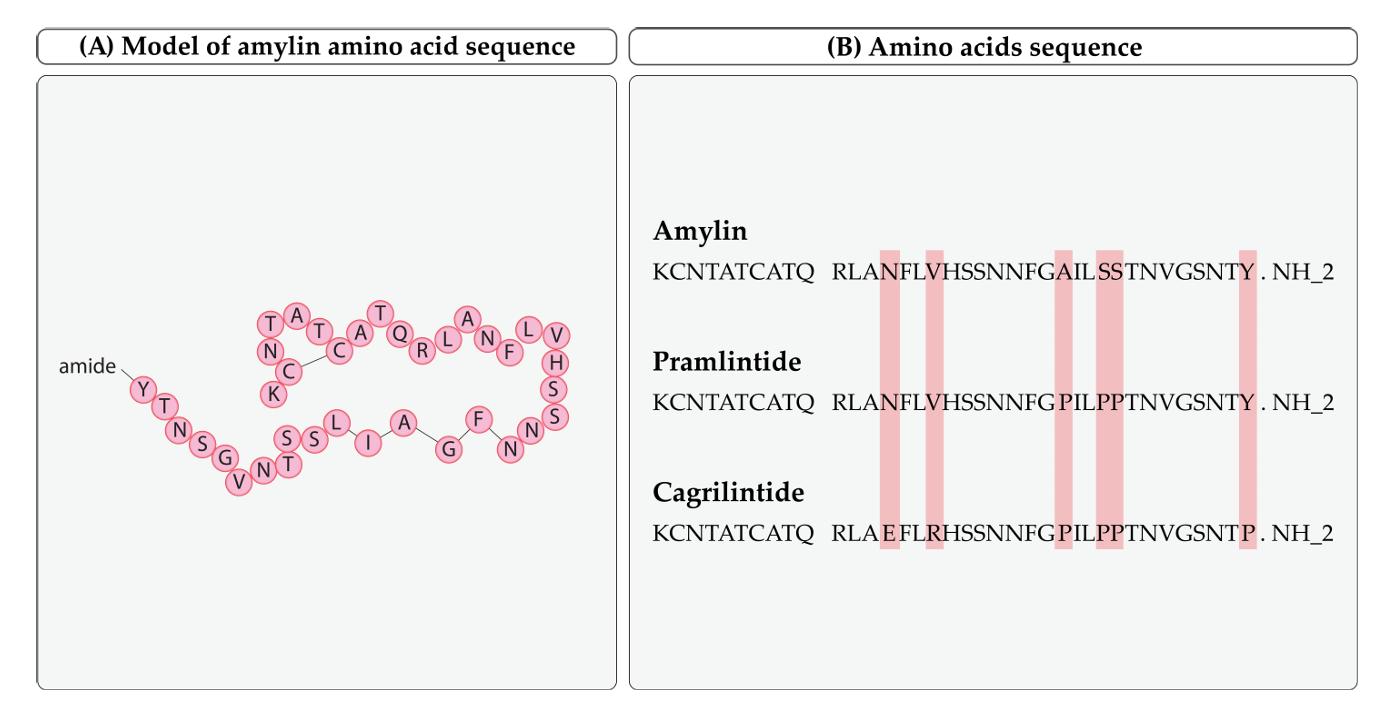 Amylin, pramlintide, and cagrilintide amino acid sequences. (Eržen S., et al., 2024)
Amylin, pramlintide, and cagrilintide amino acid sequences. (Eržen S., et al., 2024)
From July 25, 2018, to December 17, 2019, 285 individuals were screened, and 96 were randomly assigned to receive either cagrilintide or placebo, alongside semaglutide 2.4 mg. Of these, 95 were subjected to treatment (one patient in the 0.60 mg cagrilintide group was not treated) and were included in the safety and full analysis datasets. The average age was 40.6 years (SD 9.2), with 56 (59%) of 95 participants being male and 51 (54%) identifying as Black or African American. Among 566 adverse events reported in 92 individuals (69 [97%] of 71 randomized to 0.16–4.5 mg cagrilintide and 23 [96%] of 24 assigned to placebo), 207 (37%) were gastrointestinal illnesses. The majority of adverse events were of mild to moderate intensity, and the percentage of patients experiencing one or more adverse events was comparable across treatment groups. Exposure was directly proportionate to the cagrilintide dosage and did not influence the exposure or elimination of semaglutide. AUC0–168 h varied from 926 nmol × h/L to 24,271 nmol × h/L, whereas Cmax ranged from 6.14 nmol/L to 170 nmol/L with cagrilintide dosages of 0.16–4.5 mg. The AUC0–168 h varied from 12,757 nmol × h/L to 15,305 nmol × h/L, while Cmax ranged from 96.4 nmol/L to 120 nmol/L for semaglutide 2.4 mg. Cagrilintide 0.16−4.5 mg had a half-life of 159–195 hours, with a median tmax of 24–72 hours. Semaglutide 2.4 mg had a half-life of 145–165 hours, with a median tmax of 12–24 hours. The plasma clearance and volume of distribution for cagrilintide and semaglutide were comparable across the treatment groups. At week 20, mean percentage reductions in body weight were significantly greater with cagrilintide 1.2 mg and 2.4 mg compared to placebo (15.7% [SE 1.6] for cagrilintide 1.2 mg and 17.1% [1.5] for cagrilintide 2.4 mg versus 9.8% [1.2] for pooled placebo cohorts 1–5; estimated treatment difference of −6.0% [95% CI −9.9 to −2.0] for cagrilintide 1.2 mg and −7.4% [−11.2 to −3.5] for cagrilintide 2.4 mg compared to pooled placebo), as well as with cagrilintide 4.5 mg versus matched placebo (15.4% [1.3] vs 8.0% [2.2]; estimated treatment difference −7.4% [−12.8 to −2.1]), all in conjunction with semaglutide 2.4 mg. Glycaemic indicators increased across all treatment groups, irrespective of cagrilintide dosage. Hormonal alterations were analogous across the therapy cohorts.
Cagrilintide is a long-acting, acylated derivative of amylin that exhibits agonistic properties on both natural amylin and calcitonin receptors and has been studied for weight control purposes. It targets the glucagon receptor (GCGR) to enhance the feeling of fullness (satiety) and decrease food cravings. Cagrilintide has many structural changes in its peptide composition when compared to pramlintide. The proline alterations (Pro25, Pro28, and Pro29) in cagrilintide inhibit the formation of amyloid fibrils. Moreover, the Tyr37 Pro replacement enhances effectiveness. The peptide includes two modifications: Asn14 Glu, which inhibits deamination, and Val17 Arg, which enhances solubility at physiological pH and forms a helix-stabilizing salt bridge with Glu14. The incorporation of a C-20 fatty diacid via an α-glutamyl spacer prolongs the duration of action by binding to albumin.
Tirzepatide (TZP), an agonist of glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptors (GIPR/GLP-1R), has shown in clinical trials superior reductions in glucose, body weight, and triglyceride levels compared to selective GLP-1R agonists in individuals with type 2 diabetes (T2D). Diet-induced obese rats received subcutaneous doses of 3 and 10 nmol/kg of cagrilintide and tirzepatide, either individually or in combination at a submaximal dosage of 3 nmol/kg, once daily for 12 days. Daily measurements were taken of body weight and food consumption. Plasma biochemical parameters were evaluated. In comparison to tirzepatide, cagrilintide given alone shown more effectiveness in body weight loss, achieving -7.22% ± 1.80% at 3 nmol/kg and -9.61% ± 0.52% at 10 nmol/kg, while tirzepatide resulted in -2.53% ± 0.44% at 3 nmol/kg and -5.81% ± 0.86% at 10 nmol/kg. A peptide combination administered at a submaximal dosage of 3 nmol/kg each resulted in a body weight reduction of -11.0 ± 0.86% (P<0.05 compared to cagrilintide and P<0.001 compared to tirzepatide) at equivalent doses. Decreases in total food consumption were associated with weight reduction. Combination treatment shown greater efficacy in reducing ALT compared to cagrilintide (P<0.001) and tirzepatide (P<0.05), whereas plasma TG levels were significantly lower than those in the tirzepatide group (P<0.001). Combination treatment using submaximal dosages of cagrilintide and tirzepatide resulted in much better weight loss and a more substantial decrease in food consumption than monotherapy, indicating the possibility for future clinical development of this combination.
With its extended elimination half-life, the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide may be administered subcutaneously (sc) once weekly. Treatment for T2DM with once-weekly sc semaglutide has recently been authorized by both the FDA. In patients with T2D, once-weekly sc semaglutide seems to have a better weight reduction effectiveness than the other once-weekly GLP-1 RAs. A phase II dose-finding experiment recently tested semaglutide for its antiobesity effects; the results showed that once-daily sc semaglutide was more effective in reducing weight than placebo and once-daily 3.0 mg liraglutide in people who were overweight but did not have T2D.
Further research in obesity is warranted based on the clinical findings reported for the combination of cagrilintide and semaglutide, which show that cagrilintide, when added to semaglutide, may elicit an additional 7.4% weight reduction, for a total of 17.1%. A half-life of 159–195 hours was determined. There was no direct measurement of albumin binding of cagrilintide, however this supports lipidation with fatty acids as a method to extend the half-life of peptide hormones. A large body of data suggests that negatively charged fatty acid derivatives and long-acting peptides or proteins bind strongly and reversibly to albumin. Additionally, we have investigated comparable in vitro receptor experiments as described here, but with different concentrations of albumin. The results corroborate the idea that cagrilintide, like the GLP-1 analog semaglutide, is a reversible albumin binder.
Retatrutide is an innovative triple agonist targeting the glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and glucagon receptors. A 48-week phase 2 obesity trial revealed weight reductions of 22.8% and 24.2% with retatrutide dosages of 8 mg and 12 mg, respectively. Retatrutide 12 mg administered subcutaneously once weekly shown enhancements in cardiometabolic risk factors, including systolic and diastolic blood pressure, triglycerides, LDL-cholesterol, total cholesterol, HbA1c, fasting glucose, and insulin at weeks 24 and 48. Retatrutide also normalized hepatic fat in the majority of patients. For the first time, a study demonstrated that all subjects receiving either 8 mg or 12 mg of retatrutide saw a minimum weight loss of 5%, while the overall safety and tolerability profile was comparable to other GLP-1 receptor agonists. Cagrilintide is beneficial in combination treatment, but Retatrutide has remarkable efficacy as a monotherapy but may pose increased metabolic concerns.
Cagrilintide is mostly under development and investigation as a therapeutic intervention for obesity and weight control. It operates by simulating the actions of the endogenous hormone amylin, which plays a role in appetite regulation, food consumption, and stomach emptying. It facilitates weight reduction by inhibiting hunger and decelerating stomach emptying, resulting in less caloric consumption. Cagrilintide is often used in conjunction with GLP-1 receptor agonists (e.g., semaglutide) to augment the total weight reduction efficacy. In a phase 2 dose-finding experiment including individuals with overweight or obesity and hypertension or dyslipidemia without T2D, cagrilintide administered at a dosage of 2.4 mg, in conjunction with diet and exercise, led to a 10% decrease in body weight after 26 weeks; the placebo group saw a 3% reduction in body weight. The weight reduction impact of cagrilintide is more pronounced when co-administered with other medications. After 20 weeks, the co-administration of cagrilintide 2.4 mg with semaglutide 2.4 mg led to a 17% decrease in body weight; conversely, the co-administration of semaglutide 2.4 mg with placebo resulted in a 10% reduction in body weight.
A phase 2 experiment revealed that weekly cagrilintide injections resulted in a 10.8% weight reduction over 26 weeks, in contrast to 9% with liraglutide and 3% with placebo (mean age 52.3 years, with no higher age restriction). Gastrointestinal side effects and injection site responses were manageable with gradual dosage titration; nonetheless, weariness seems to be a distinctive adverse effect of cagrilintide compared to GLP-1 receptor agonists.
Combining cagrilintide with semaglutide forms CagriSema. Over the course of 32 weeks, a phase two study included 58 people (with no maximum age restriction) found that those taking CagriSema had a 15.6% mean decrease in body weight, compared to 5.1% with semaglutide and 8.1% with cagrilintide in monotherapy. Also, the average decreases in HbA1c were larger with CagriSema. With a minimum age of 55 years and no higher limit, REDEFINE is an ongoing phase 3 study that acts as a market assessment for both CagriSema and cagrilintide. Of the participants, 60% were randomly assigned to receive CagriSema, 10% to cagrilintide, 10% to semaglutide, and 20% to a placebo.
Related Peptides at Creative Peptides
| CAT# | Name | CAS | Price |
|---|---|---|---|
| R2067 | Cagrilintide | 1415456-99-3 | Inquiry |
| 10-101-158 | Albiglutide | 782500-75-8 | Inquiry |
| 10-101-16 | Exenatide | 141732-76-5 | Inquiry |
| 10-101-59 | Liraglutide | 204656-20-2 | Inquiry |
| 10-101-325 | Semaglutide | 910463-68-2 | Inquiry |
FAQ
1. Is cagrilintide the same as semaglutide?
Cagrilintide is a long-acting amylin analog, whereas semaglutide is a well-known glucagon-like peptide-1 (GLP-1) receptor agonist.
2. What is the difference between semaglutide and Cagrilintide?
Cagrilintide is an amylin analog. Amylin is a peptide that is co-secreted from the pancreas with insulin and serves as a satiety signal. It works slightly differently from semaglutide since the glucagon-like peptide is released from the gut which serves to decrease gastric emptying and to prevent glucose spikes.
References