An important homeostatic regulator, insulin-like growth factor-1 (IGF-1), is a single-chain peptide (7.5 kDa) that plays a role in a wide variety of physiological, anabolic, and metabolic processes throughout the body. About ninety-nine percent of IGF-1 in circulation is bound to one of six known binding proteins; the most prevalent of these is insulin-like growth factor binding protein-3 (IGFBP-3). Although the functional significance of these binding proteins is not fully known, other binding proteins are more abundant in tissues, with IGFBP-2, -4, and -5 being more strongly expressed in the brain. After proteases break down IGFBP, the physiologically active 'free' version of IGF-1 or a combination of it with one of the smaller IGFBPs may exit the bloodstream. Despite the fact that almost every tissue in the body is capable of producing IGF-1 in a paracrine or autocrine fashion, the vast majority of the binding proteins and around 75% of the circulating IGF-1 in humans are made in the liver. Insulin has the ability to enhance hepatic IGF-1 secretion by upregulating GH receptors, while GH primarily controls hepatic IGF-1 synthesis.
The cellular effects of insulin-like growth factor 1 are transmitted by means of IGF-1 tyrosine-kinase membrane receptor activation. When IGF-1 binds to its surface receptor (IGF-1R), it sets off a series of events within the cell that lead to the activation of several signaling pathways, including the PI3K-Akt pathway, the mitogen-activated protein kinase (MAPK) signaling cascade, and the phosphorylation of insulin receptor substrate molecules. The activations in question exert regulatory effects on the mTOR activity and the FoxO translocation, both of which are recognized to have a role in cellular aging.
The IGF-1 gene is on the long arm of chromosome 12q23-23. The human IGF-1 gene consists of six exons, including two leader exons, and has two promoters. The IGFs are members of a family of insulin related peptides that include relaxin and several peptides isolated from lower invertebrates. IGF-1 is a small peptide consisting of 70 amino acids with a molecular weight of 7649 Da. Similar to insulin, IGF-1 has an A and B chain connected by disulphide bonds. The C peptide region has 12 amino acids. The structural similarity to insulin explains the ability of IGF-1 to bind (with low affinity) to the insulin receptor.
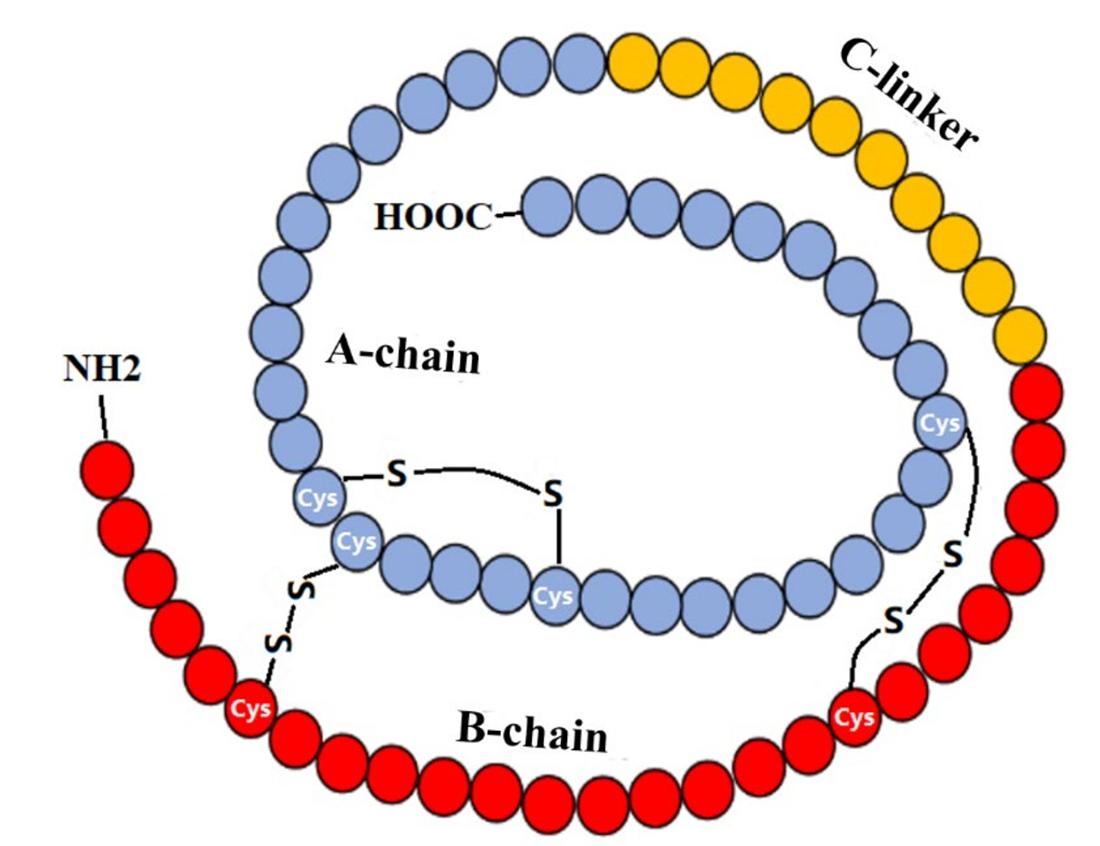 Scheme of IGF-1 Structure. (Ge L., et al., 2022)
Scheme of IGF-1 Structure. (Ge L., et al., 2022)
The insulin-like growth factors (IGF1 and IGF2) are mitogenic peptides that regulate vertebrate growth. The IGFs are members of the evolutionarily ancient insulin-like family of peptides, found throughout the metazoans. Conserved features of the insulin-like peptides include regulation by nutritional status, and roles in nutrient metabolism, growth, development, reproduction, and aging. IGF1 is thought to have arisen from insulin during the transition from chordates to primitive vertebrates, and IGF2 to have arisen from IGF1 in the common ancestor of the bony and cartilaginous fishes.
Liver-derived circulating IGF1 plays an essential role in postembryonic mammals as the primary mediator of GH-dependent growth. As developed in the somatomedin hypothesis, pituitary GH stimulates liver production of IGF1, which feeds back to inhibit GH secretion in the GH/IGF endocrine axis. In mice, both endocrine and local IGF1 play biologically significant roles in growth regulation. Nutritional and metabolic status regulate the GH/IGF axis through direct effects on the liver, and by modulating liver sensitivity to GH. In catabolic states such as fasting and disease, liver production of IGF1 becomes resistant to stimulation by GH. Metabolically responsive hormones link the anabolic/catabolic state of the animal to liver GH sensitivity. Insulin directly increases hepatocyte IGF1 production, and strongly increases hepatocyte responsiveness to GH in vivo, and in mammalian and avian primary hepatocyte culture.
In all vertebrate classes, IGF2 is highly expressed during embryogenesis, and stimulates growth in embryonic tissues. In contrast, IGF1 expression is low during embryogenesis and increases with the onset of postnatal GH-dependent growth. It is thought that liver IGF2 production is not strongly stimulated by GH or other hormones in postnatal mammals. The lack of a clear picture of the regulation and function of endocrine IGF2 in postnatal mammals may be due to the central role of IGF2 in the development of the placenta. IGF2 is a principal regulator of the size and exchange capacity of the placenta. The parent–offspring evolutionary conflict theory predicts that this role as a mediator of maternal-fetal resource partitioning has resulted in selection on IGF2 and divergence of IGF2 regulation and function.
IGF-1 attaches to a tyrosine kinase receptor 34 called the insulin-like growth factor 1 receptor (IGF-1R). When it comes to binding affinity, IGF-1 has a leg up over IGF-2. A series of downstream signal transduction pathways, including Ras/Raf/ERK and PI3K/Akt/mTOR, are known to be involved in cell growth, proliferation, and cancer, and they are initiated by IGF-1R. The liver acts as an endocrine hormone, producing the bulk of IGF-1 that is detected in circulation. When autocrine or paracrine processes are in work, other organs will also create IGF-1. Extensive research points to IGF-1 and IGF-1R as critical for cancer cell proliferation and maintenance. While other hormones do play a role, growth hormone (GH) has a far larger role in controlling IGF-1 gene expression. On the other hand, IGF-1 that is produced locally via autocrine or paracrine processes has the potential to promote the formation of some malignancies. GH, which has mitogenic and proliferative characteristics, controls the aging-related variability in circulation IGF-1 levels, which peak around puberty and then decrease with age. The growth-stimulating effects of IGF-1 are reliant on GH in certain cell types, but not in others; for instance, cartilage cells. Also, GH insufficiency is the most prevalent condition among children cancer survivors, and there are worries that using it to treat cancer survivors might raise the incidence of SPCs. The oncogene classification does not apply to IGF-1 despite its antiapoptotic, cell survival, and transforming properties.
Up to this point, research has shown conflicting results on the involvement of IGF-1 in the progression of breast cancer. Women with breast cancer had greater circulating levels of IGF-1 than women without the disease, according to an early case-control research that was carried out in 1993. Multiple epidemiological studies have now shown that elevated IGF-1 levels in the blood are linked to a greater probability of developing breast cancer. This could happen because there is some evidence that links elevated IGF-1 levels to a faster rate of early carcinogenesis. In more recent times, three meta-analyses found that IGF-1 was positively associated with breast cancer risk in premenopausal women, but not in postmenopausal mothers. In an effort to shed light on these findings, Schernhammer's group conducted a study that found premenopausal women with high levels of IGF-1 were more likely to have higher levels of IGF-1R activation in their mammary epithelial cells. This activation enhances the survival of these cells in the face of accumulating DNA damage, which in turn facilitates stepwise carcinogenesis. Possible interactions with other hormones, such as estrodial and growth hormone, or the significance of IGF-1 levels in early life in younger women may be suggested by these findings. Yet, a large prospective research that combined two Swedish cohorts came to the opposite conclusion: circulating IGF-1 was not associated with an increased risk of breast cancer, independent of menopausal state. Why there is a difference in the results of the studies is not apparent. However, variations in results can be explained by factors such as the subtype of breast cancer, the timing of blood samples, or the patient cohort. Since blood samples are taken to evaluate IGF-1 levels before the clinical diagnosis of cancer, prospective studies have an advantage over retrospective ones. This is because reverse causation, where an undiscovered malignancy's effects on IGF-1 levels are considered less probable, is less likely to be a factor.
The link between IGF-1 and lung cancer has been the subject of research up to this point. Multiple investigations have failed to find any correlation between elevated levels of circulating IGF-1 and an increased risk of lung cancer. The incidence of lung cancer was positively associated with IGF-1 in one case-control research (OR: 2.06; 95% CI: 1.19-3.56). Cigarette smoking did not affect the plasma levels of insulin-like growth factor 1 and 2 according to this investigation. The primary protein in blood that binds IGF-1 is IGFBP-3. It is widely believed that IGFBP-3 functions as a tumor suppressor gene by decreasing IGF-1's capacity to stimulate cell survival and proliferation. Comparing the highest and lowest quartiles of IGFBP-3 in a Chinese prospective research revealed a decreased risk of lung cancer (OR: 0.50, 95% CI: 0.25-1.02), even though epidemiological studies generally did not find a connection for IGF-1. In addition, many meta-analyses have shown the opposite to be true about the relationship between IGFBP-3 and lung cancer risk.
Many studies have looked at the link between IGF-1 and prostate cancer, and they always find a positive one. Research on the possible link between elevated circulating IGF-1 levels and an increased risk of prostate cancer dates back to 1993. Earlier research did not find any evidence linking IGF-1 to an increased risk of prostate cancer. In a case-control research, Mantzoros's group looked at the first strong positive correlation between IGF-1 and prostate cancer. An odds ratio of 1.91 (95% CI: 1.00-3.73), calculated by comparing males with prostate cancer to healthy controls, was seen for every 60 ng/mL increase in circulating IGF-1 levels. In addition, the scientists noted that the absence of a correlation between IGF-1 and benign prostatic hyperplasia lends credence to this link.
Research has consistently shown a favorable correlation between IGF-1 levels and colorectal cancer. A higher risk of colorectal cancer was linked to elevated levels of insulin-like growth factor-1 in the blood, according to preliminary research conducted in the late 1990s. Nomura et al. looked studied the link between IGF-1 and rectal cancer and colon cancer independently. Individuals with IGF-1 levels in the third quartile (137-174 ng/mL) or fourth quartile (IGF-1 > 174 ng/mL) were shown to have a greater risk of colon cancer compared to the controls, with odds ratios of 2.2 and 1.8, respectively. The link between IGF-1 and colon cancer was not found. On the other hand, elevated levels of circulating IGF-1 were associated with a reduced risk of rectal cancer in a different research. The authors speculated that this may be because rectal cancer often manifests before colon cancer does, hiding the link between the two. The results of two nested case-control studies, however, failed to demonstrate a statistically significant link between IGF-1 and the likelihood of developing colorectal cancer.
In a model of experimental colitis generated by oral administration of 2% dextran sulphate sodium (DSS) for ten days, inflammation in the mucosa and submucosa was decreased after 7 days of IGF-1 therapy. Research conducted by Yu's group revealed that the release of high-mobility group box 1 (HMGB1) is reduced by IGF-1 therapy, which in turn reduces ox-LDL-induced inflammation. A powerful activator of tissue damage and inflammation, HMGB1 is a non-histone nuclear protein. Intriguingly, HMGB1 may go outside of cells and serve a twofold purpose: first, as a cytokine produced by inflamed cells in response to pathogen activation; and second, as a protein secreted passively by necrotic cells, which activates nuclear factor kappa B (NF-kB), setting off inflammatory responses. Consistent with this, Guo's group highlights IGF-1's anti-inflammatory function. Myocardium cell apoptosis was reduced, and pro-inflammatory cytokine gene expression and protein synthesis were suppressed by IGF-1 in their post-myocardial infarction investigation. Furthermore, an estrogen-deficient mouse's skin wounds healed more quickly after receiving local injections of exogenous IGF-1. According to Johannesson's group, IGF-1 increases the frequency of Foxp3+ Treg cells and decreases immune cell infiltration in an afflicted region, therefore suppressing allergic contact dermatitis in a Treg cell-specific way. It has been shown that the anti-inflammatory cytokineIL-10 is upregulated after IGF-1 treatment. Possible treatment implications for other inflammatory disorders, such as inflammatory bowel disease (IBD), stem from the study's finding that IGF-1 directly promotes the proliferation of regulatory T cells. Potentially related to malabsorption, malnutrition, and GH/IGF-1 resistance, growth retardation in children with IBD may be treatable with IGF-1 and its analogs.
Given IGF-1's strong stimulatory impact on glucose absorption, the medication might potentially replace conventional diabetic therapy in some clinical contexts. In individuals suffering from severe insulin resistance, the use of IGF-1 leads to an improvement in metabolic regulation. Additionally, glucose tolerance, hyperinsulinemia, and hypertriglyceridemia are all improved in individuals with type II (non-insulin-dependent) diabetes who take IGF-1. Progression of certain neoplasms and diabetic problems including nephropathy and retinopathy might be among the undiscovered consequences linked to long-term IGF-1 treatment. Some individuals with severe insulin resistance who have metabolic crises may benefit most from insulin-like growth factor-1, and it may also be an effective supplementary medication for the treatment of diabetes. However, further information is required to assess the advantages and disadvantages of IGF-1 use in diabetes and other conditions linked to reduced insulin activity.
IGF1 is involved in nutrient-sensing response to insulin, as liver-specific IGF1R-deficient mice develop muscle insulin resistance and IGF1 treatment has been shown to improve insulin action in small clinical studies. Recent studies identified that in addition to macrophages, adipocytes also express IGF1 and that local, adipose-derived IGF1 influences adaptation to metabolic stress. We showed that macrophages activated by IL-4 into a M2-like polarized state secrete higher levels of IGF1 and express IGF1R, suggesting auto/paracrine role of this classical hormone in macrophage function. In animals given a high-fat diet, insulin resistance developed after myeloid cell IGF1 receptor ablation decreased phagocytosis, increased macrophages in adipose tissue, increased adiposity, decreased energy expenditure, and impaired insulin secretion. Studies on the adipose macrophage phenotype in MIKO mice, which are obese and lacking myeloid IGF1R, found a decrease in transcripts linked to the activation of M2-like macrophages. In addition, MIKO mice infected with the parasite Nippostrongylus brasiliensis showed normal insulin sensitivity but a delayed recovery from infection. Cold exposure did not cause adipose tissue macrophages from either control or MIKO animals to enter an obvious M2-like state or to produce tyrosine hydroxylase. These findings demonstrate that the macrophage-activation phenotype is influenced by IGF1 signaling.
Neuritic outgrowth (an increase in the number of dendrites, axonal cones, and synapses), neuron progenitor proliferation and differentiation, and post-injury circumstances are maxima in the harmonic production of IGF-1. Cultured neural stem cells (NSC) express both IGF-1 and IGF-IR, and in response to IGF-1, cultured NSC differentiate into neurons or oligodendrocytes, but whether or not IGF-1 may impact NSCs is still up for discussion. Somewhat surprisingly, these mechanisms include more than only IGF-1 generated by neurons. Twenty years ago, researchers discovered that systemic IGFs may penetrate the BBB. They did this by injecting tagged IGFs into adult rats' common carotid arteries; further analyses revealed that these IGFs had also entered the brain's choroid plexus, median eminence, arterioles, and parenchyma. Indeed, prior research established that the BBB's constituent cells, the brain capillary endothelial cells, have IGF-1 receptors and are responsible for bringing circulating IGFs into the central nervous system. Newer research by Torres-Aleman's group deftly proved that glutamate release at active sites initiates this process, which in turn triggers two secondary processes: vasodilation to enhance local serum IGF-1 availability and an increase in matrix metalloprotease 9 activity, along with cleavage from IGFBP-3. Transcytosis, a process reliant on the endothelium transporter lipoprotein related receptor 1, increases the local availability of free serum IGF-1 as a consequence of these processes. This study has the potential to clarify earlier findings that point to IGF-1 produced from the liver as a key player in controlling the excretion of Aβ levels in the brain and how it might be related to Alzheimer's disease. The lack of a link between serum and cerebrospinal fluid IGFs concentrations is interesting, but it suggests that systemic IGFs do not provide a substantial amount of IGFs to the central nervous system.
Approximately 75% of the insulin-like growth factor I (IGF-1) levels in the blood come from the liver, which is the primary site of GH stimulation on hepatocytes. Interestingly, there is less information available on the local effects of IGF-1 in the liver, perhaps because there are so few IGF-1 receptors on the hepatocytes membrane. This is despite the fact that IGF-1 produced from the liver has endocrine effects on tissues outside of the liver. On the other hand, nonparenchymal cells do have IGF-1 receptors, and in vitro studies have shown that IGF-1 increases DNA synthesis and HGF production in hepatic stellate cells. It follows that IGF-1 produced by the liver cannot promote liver development in adults due to the absence of IGF-I receptors on hepatocytes. Therefore, it is probable that an unsuppressed GH production directly stimulated the abnormally large livers seen in mice with liver-specific IGF-1 deficiency, rather than reduced hepatic development. Consistent with this observation, transgenic mice overexpressing GH showed an uneven increase in liver size, although this effect was less pronounced in mice overexpressing IGF-1. Mice without the GH receptor also had a smaller relative liver weight.
Immune system reconstitution, thymus growth and function, and hematopoiesis are among areas where IGF-1 is known to have a role. There have also been reports about the function of IGF-1 on several types of immune cell lines. When it comes to the maturation and operation of T lymphocytes, both IGF-1 play critical roles. Some studies have shown that IGF-1 can inhibit IL-2-dependent lymphocyte growth and function, but more specifically, it can enhance the number of CD4+CD8+ immature T cells in the thymus and spleen of rats, as well as T cell survival, proliferation, chemotaxis, and maturation. The issue of whether IGF-1 can regulate the lifelong filling of T-cell compartments is yet unanswered. But because IL-7 is crucial to that process and IGF-1 enhances IL-7's effects on pro-B cell proliferation, it's probable that it has a similar effect on T cells.
An essential organ for the effects of GH and IGF-1 is the cardiovascular system. Evidence suggests that endothelial cells, smooth muscle in the aorta, and the heart all express IGF-1 and its receptor; these tissues are all more responsive to IGF-1 than insulin. Also, in reaction to GH, cardiac IGF-1 synthesis goes up. As a result, GH's direct activities and IGF-1's endocrine or autocrine/paracrine effects on the cardiovascular system are both possible. An increase in NO release from the endothelium may facilitate IGF-1's powerful vasodilatory effects, according to previous research. There is mounting evidence that vascular disorders including atherosclerosis and restenosis are influenced by inadequate IGF-1 levels.
In recent years, there has been a correlation between low circulating IGF-1 levels and an increased risk for CVD in people. Research has shown that low levels of IGF-1 in the blood are linked to coronary artery disease that has been confirmed through angiograms. This suggests that low IGF-1 levels may be a predictor of fatal ischemic heart disease, an increased risk of congestive heart failure and ischemic stroke in the elderly, and a worse prognosis for recovery following an acute myocardial infarction. Furthermore, in healthy centenarians, there is a relationship between circulating IGF-1 levels and coronary flow reserve as well as effective cardiovascular aging. The existing evidence from transgenic mice that lack IGF-I from the liver makes it clear that this shortage speeds up the aging process in the cardiovascular system. Experimental aortic constriction substantially reduces cardiac myocyte contractility and leads to a defective compensatory hypertrophic response. Similar to the aging phenotype, this animal model shows a dysregulation of Nrf2-dependent antioxidant responses in the vasculature, which leads to increased oxidative stress, endothelial dysfunction, and apoptosis.
Related Peptides at Creative Peptides
| CAT# | Product Name | M.W | Molecular Formula | Price |
|---|---|---|---|---|
| 10-101-260 | IGF-1 LR3 | 9117.6 | C400H625N111O115S9 | Inquiry |
| I01001 | IGF-I Analog | 1249.52 | C55H88N14O15S2 | Inquiry |
| I01002 | IGF-I (1-3) | 301.3 | C12H19N3O6 | Inquiry |
| I01003 | IGF-II (33-40) | 1003.13 | C38H74N20O12 | Inquiry |
| I01004 | IGF-I (24-41); Insulin-like Growth Factor I (24-41) | 2017.2 | Inquiry | |
| I01005 | IGF-I (30-41); Insulin-like Growth Factor I (30-41) | 1266.3 | Inquiry | |
| M34140607H | IGF-1 DES | 7371.4 | C319H501N91O96S7 | Inquiry |
| R1440 | IGF-I (24-41) TFA | 2131.18 | C₈₈H₁₃₃N₂₇O₂₈.C₂HF₃O₂ | Inquiry |
| R1441 | IGF-I (30-41) TFA | 1380.36 | C₅₁H₈₃N₁₉O₁₉.C₂HF₃O₂ | Inquiry |
| R1442 | IGF-I 24-41 | 2017.16 | C₈₈H₁₃₃N₂₇O₂₈ | Inquiry |
| R1443 | IGF-I 30-41 | 1266.34 | C₅₁H₈₃N₁₉O₁₉ | Inquiry |
| 10-101-261 | LONG R3 IGF-1 (human) | 9111.45 | C400H619N111O115S9 | Inquiry |
| I01008 | rec IGF-I (human) | Inquiry | ||
| I01009 | rec IGF-II (1-67) (human) | 7469.46 | Inquiry |
References