Phosphorylation is a widespread post-translational modification that occurs as a result of esterification of amino acid side chains in peptides by the addition of a strongly negatively charged phosphate group, thereby altering the protein's conformation, activity, and ability to interact with other molecules, and plays an important role in the regulation of many biological processes, such as signaling, gene expression, and cell division. Phosphorylated peptides are analyzed by using liquid chromatography to separate components of the enzymatic peptide mixture to reduce sample complexity, then enriching the modified peptides with high-quality phosphorylation-modified antibodies and biomaterials, and finally uploading the samples to liquid chromatography-tandem mass spectrometry (LC-MS/MS) for analysis and quantification.
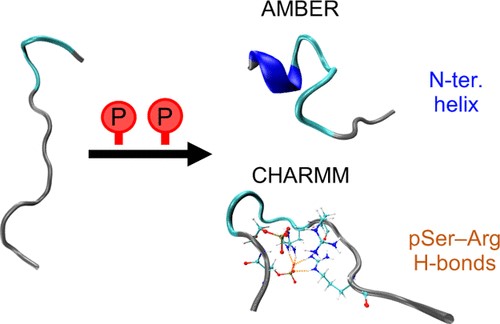 Fig. 1. Phosphorylation of disordered peptides (J. Chem. Theory Comput. 2020, 16(3): 1924-1935).
Fig. 1. Phosphorylation of disordered peptides (J. Chem. Theory Comput. 2020, 16(3): 1924-1935).
Phosphorylated proteins are categorized into four groups, namely O-phosphates, N-phosphates, acylphosphates and S-phosphates.
The amino acid residues at which phosphorylation occurs vary from species to species. In eukaryotes, phosphorylation occurs mainly on residues such as serine, threonine, and tyrosine. In bacteria, proteins are phosphorylated mainly through residues such as aspartic acid, glutamic acid, and histidine. Some proteins can be phosphorylated in both prokaryotes and eukaryotes, and their phosphorylation sites are usually arginine, lysine, and cysteine residues.
Phosphorylation of peptides changes their molecular mass accordingly, and mass spectrometry can accurately determine the molecular mass of peptides. On the other hand, the amount of post-translationally modified proteins in a sample is usually low and has a wide dynamic range, so it is necessary to enrich the modified peptides before mass spectrometry detection.
Titanium dioxide enrichment is currently the most widely used metal oxide enrichment method for phosphopeptides. Titanium dioxide (TiO2) is positively charged and binds specifically to the negatively charged phosphate groups on the phosphorylated modified peptide fragments in the peptide sample. After the non-phosphorylated peptides were removed by washing buffer, the phosphorylated peptides bound to TiO2 were eluted with alkaline eluent to achieve specific enrichment of phosphorylated peptides.
(1) Analysis of phosphopeptide ions by MALDI-TOF MS
MALDI-TOF MS is often used to analyze complete peptide profiles. Thus, if the identification is obtained by peptide spectrum matching of the protein, then the phosphopeptide can be simply identified by detecting whether the 79.983 relative molecular mass migration occurs in the resulting peptide spectrum versus the theoretical peptide spectrum. In addition, treatment of the sample with alkaline phosphatase can help identify the phosphorylated peptide by relocating the phosphorylated mass-migrated peptide back to the theoretically predicted position. If the peptide contains only one possible phosphorylation site and either contains the corresponding target site of a certain kinase or the chemical modification of the phosphorylated residue has been characterized, the site of the phosphorylation modification may be accurately identified.
(2) Fragment ion analysis
In phosphorylation proteomics, phosphopeptides produce specific ionic fragments, such as H2PO4-, PO3-, PO2-, etc., with relative molecular masses of 97, 79, and 63, etc. The presence of these ions in the mass spectrometry therefore indicates that a phosphopeptide is present in the sample. In addition, the peptide backbone fragmentation technique may allow the peptide chain sequence to be reconfigured, and if the sequence contains phosphorylated residues, they can be localized unambiguously.
Reference